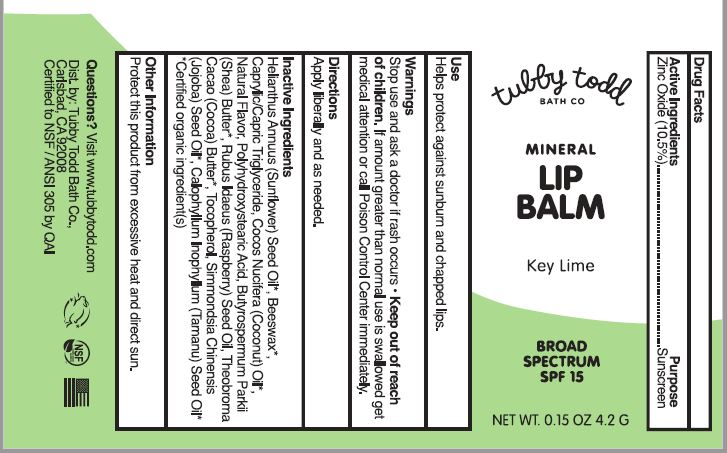
MINERAL LIP BALM SPF-15- zinc oxide lipstick
Mineral Lip Balm by
Drug Labeling and Warnings
Mineral Lip Balm by is a Otc medication manufactured, distributed, or labeled by TUBBY TODD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Helianthus Annuus (Sunflower) Seed Oil*, Beeswax*, Caprylic/Capric Triglyceride, Cocos Nucifera (Coconut) Oil*, Natural Flavor*, Polyhydroxystearic Acid, Butyrospermum Parkii (Shea) Butter*, Rubus Idaeus (Raspberry) Seed Oil, Theobroma Cacao (Cocoa) Butter*, Tocopherol, Simmondsia Chinensis (Jojoba) Seed Oil*, Calophyllum Inophyllum (Tamanu) Seed Oil*
*Certified organic ingredient(s) - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MINERAL LIP BALM SPF-15
zinc oxide lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 73088-105 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 10.5 g in 100 g Inactive Ingredients Ingredient Name Strength SUNFLOWER OIL (UNII: 3W1JG795YI) YELLOW WAX (UNII: 2ZA36H0S2V) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) COCONUT OIL (UNII: Q9L0O73W7L) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SHEA BUTTER (UNII: K49155WL9Y) RUBUS IDAEUS SEED (UNII: M3CL7US2ZG) TOCOPHEROL (UNII: R0ZB2556P8) COCOA BUTTER (UNII: 512OYT1CRR) JOJOBA OIL (UNII: 724GKU717M) TAMANU OIL (UNII: JT3LVK84A1) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73088-105-11 4.2 g in 1 TUBE; Type 0: Not a Combination Product 05/21/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/21/2019 Labeler - TUBBY TODD (052545299)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.