REYVOW (lasmiditan) tablets, for oral use, [CV in USA] - FOR EXPORT ONLY Initial U.S. Approval: 2020
Reyvow by
Drug Labeling and Warnings
Reyvow by is a Prescription medication manufactured, distributed, or labeled by Eli Lilly and Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
REYVOW- lasmiditan tablet
Eli Lilly and Company
----------
REYVOW (lasmiditan) tablets, for oral use, [CV in USA] - FOR EXPORT ONLY
Initial U.S. Approval: 2020
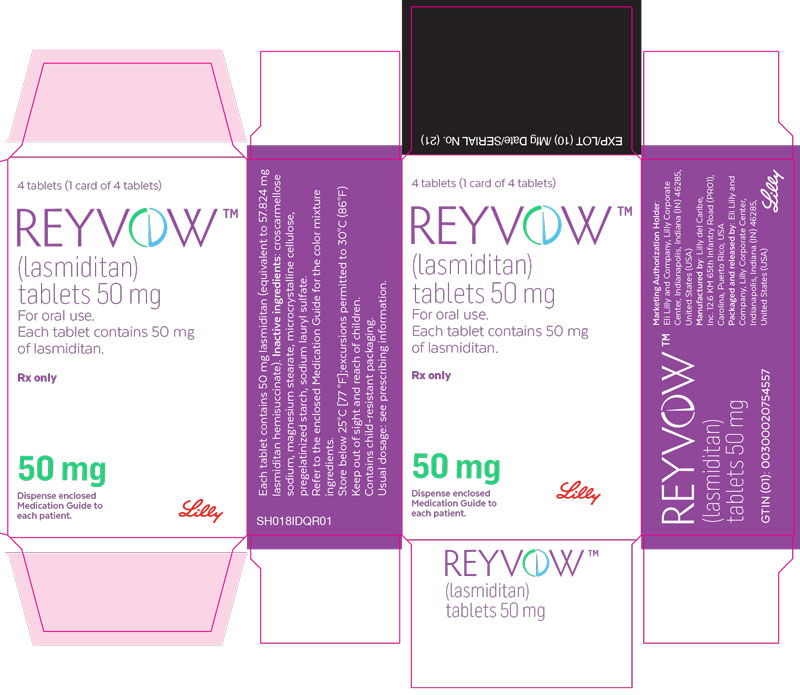
PACKAGE LABEL - REYVOW 50 mg 4ct carton - FOR EXPORT ONLY
Packaging Image for NDC: 0002-0265-04
4 tablets (1 card of 4 tablets)
REYVOW™
(lasmiditan)
tablets 50 mg
For oral use.
Each tablet contains 50 mg of lasmiditan.
Rx only
50 mg
Dispense enclosed Medication Guide to each patient.
Lilly
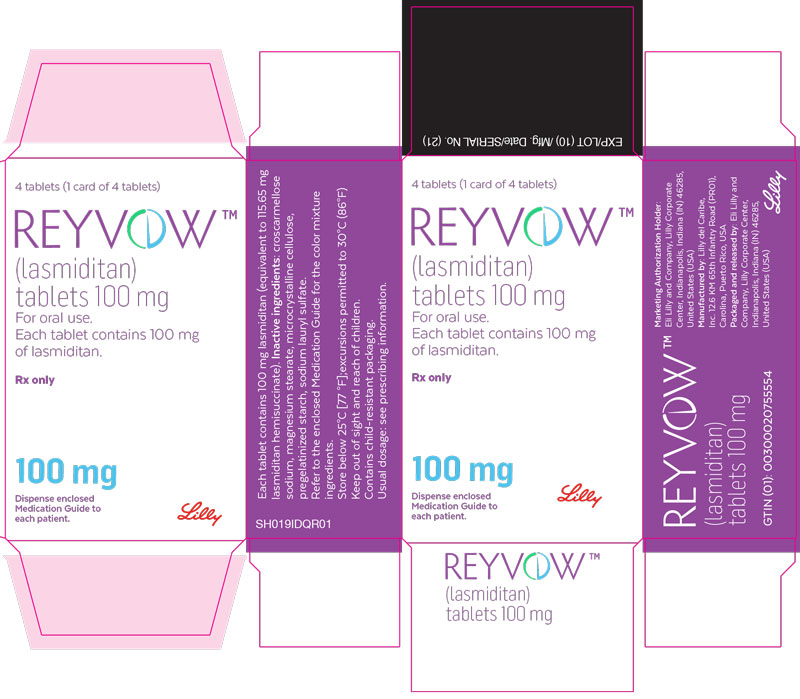
PACKAGE LABEL - REYVOW 100 mg 4ct carton - FOR EXPORT ONLY
Packaging Image for NDC: 0002-0632-04
4 tablets (1 card of 4 tablets)
REYVOW™
(lasmiditan)
tablets 100 mg
For oral use.
Each tablet contains 100 mg of lasmiditan.
Rx only
100 mg
Dispense enclosed Medication Guide to each patient.
Lilly
| REYVOW
lasmiditan tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| REYVOW
lasmiditan tablet |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Eli Lilly and Company (006421325) |
Trademark Results [Reyvow]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REYVOW 88035791 not registered Live/Pending |
Eli Lilly and Company 2018-07-12 |
 REYVOW 87446502 not registered Live/Pending |
Eli Lilly and Company 2017-05-11 |
 REYVOW 86926214 not registered Dead/Abandoned |
ELANCO US INC. 2016-03-02 |
 REYVOW 85685418 not registered Dead/Abandoned |
Eli Lilly and Company 2012-07-24 |
 REYVOW 85042374 not registered Dead/Abandoned |
Eli Lilly and Company 2010-05-19 |
 REYVOW 77141452 not registered Dead/Abandoned |
Eli Lilly and Company 2007-03-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.