GSD GOOD HYGIENE DEFENSE WET WIPES- benzalkonium chloride cloth
JoCo Sales & Marketing, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
GSD Good Hygiene Defense Wet Wipes
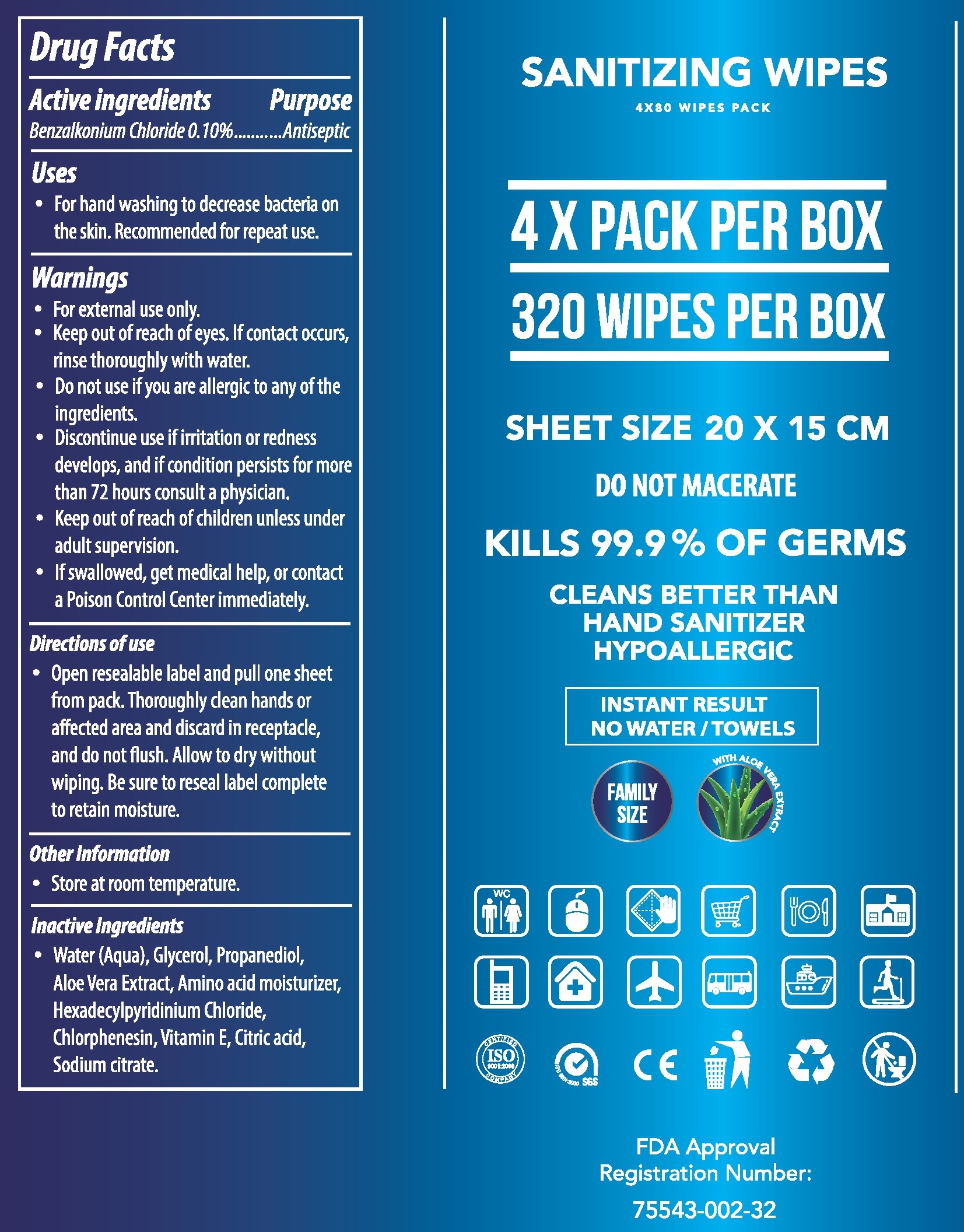
Active ingredients
Benzalkonium Chloride 0.10%
Uses
- For hand washing to decrease bacteria on the skin. Recommended for repeat use.
Warnings
- For external use only.
- Keep out of reach of eyes. If contact occurs, rinse thoroughly with water.
Do not use
- if you are allergic to any of the ingredients.
- Discontinue use if irritation or redness develops, and if condition persists for more than 72 hours consult a physician.
Keep out of reach of children
- unless under adult supervision.
- If swallowed, get medical help, or contact a Poison Control Center immediately.
Directions of use
- Open resealable label and pull one sheet from pack. Thoroughly clean hands or affected area and discard in receptacle, and do not flush. Allow to dry without wiping. Be sure to reseal label complete to retain moisture.
Other Information
- Store at room temperature.
Inactive Ingredients
- Water (Aqua), Glycerol, Propanediol, Aloe Vera Extract, Amino acid moisturizer, Hexadecylpyridinium Chloride, Chlorphenesin, Vitamin E, Citric acid, Sodium citrate.
Package Labeling:20ct

Package Labeling:80ct

Package Labeling: 77784-001-21, 200ct


77784-001-32 package label
80 wipes in a bag, 4 bags in a box. 320 Wipes total.

800ct Package label


GSD GOOD HYGIENE DEFENSE WET WIPES
benzalkonium chloride cloth |
| Product Information |
| Product Type | HUMAN OTC DRUG | Item Code (Source) | NDC: 77784-001 |
| Route of Administration | TOPICAL |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) | BENZALKONIUM CHLORIDE | 1 mg in 1 g |
|
|
|
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 77784-001-20 | 20 in 1 BAG | 06/04/2020 | 07/01/2022 |
| 1 | | 5.15 g in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) | | |
| 2 | NDC: 77784-001-80 | 80 in 1 BAG | 06/04/2020 | 07/01/2022 |
| 2 | | 5.15 g in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) | | |
| 3 | NDC: 77784-001-21 | 200 in 1 BAG | 07/01/2020 | 07/01/2022 |
| 3 | | 5.15 g in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) | | |
| 4 | NDC: 77784-001-32 | 4 in 1 BOX | 07/20/2020 | 07/01/2022 |
| 4 | | 80 in 1 BAG | | |
| 4 | | 5.15 g in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) | | |
| 5 | NDC: 77784-001-81 | 800 in 1 CANISTER | 07/16/2020 | 07/01/2022 |
| 5 | | 0.04605 g in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) | | |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part333E | 06/04/2020 | 07/01/2022 |
|
| Labeler - JoCo Sales & Marketing, Inc.
(157016291)
|







