KEVEYIS- dichlorphenamide tablet
Keveyis by
Drug Labeling and Warnings
Keveyis by is a Prescription medication manufactured, distributed, or labeled by Taro Pharmaceuticals U.S.A., inc., Taro Pharmaceutical Industries, Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use KEVEYIS™ safely and effectively. See full prescribing information for KEVEYIS™.
KEVEYIS™ (dichlorphenamide) tablets, for oral use
Initial U.S. Approval: 1958RECENT MAJOR CHANGES
INDICATIONS AND USAGE
KEVEYIS™ is an oral carbonic anhydrase inhibitor indicated for the treatment of primary hyperkalemic periodic paralysis, primary hypokalemic periodic paralysis, and related variants (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 50 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypersensitivity / Anaphylaxis / Idiosyncratic reactions: discontinue KEVEYIS™ at the first appearance of skin rash or any sign of immune-mediated or idiosyncratic adverse reaction (5.1)
- Hypokalemia: baseline and periodic measurement of serum potassium are recommended; if hypokalemia develops or persists, consider reducing the dose or discontinuing KEVEYIS™ (5.3)
- Metabolic acidosis: baseline and periodic measurement of serum bicarbonate are recommended; if metabolic acidosis develops or persists, consider reducing the dose or discontinuing KEVEYIS™ (5.4)
- Falls: consider reducing the dose or discontinuing KEVEYIS™ in patients who experience falls (5.5)
ADVERSE REACTIONS
Most common adverse reactions (incidence at least 10% and greater than placebo) include paresthesias, cognitive disorder, dysgeusia, and confusional state (6)
To report SUSPECTED ADVERSE REACTIONS, contact Taro at 1-866-923-4914, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2015
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity / Anaphylaxis / Idiosyncratic Reactions
5.2 Concomitant Use of Aspirin
5.3 Hypokalemia
5.4 Metabolic Acidosis
5.5 Falls
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Aspirin and Salicylates
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Initiate dosing at 50 mg twice daily. The initial dose may be increased or decreased based on individual response, at weekly intervals (or sooner in case of adverse reaction). The maximum recommended total daily dose is 200 mg.
Primary hyperkalemic periodic paralysis, primary hypokalemic periodic paralysis, and related variants are a heterogeneous group of conditions, for which the response to KEVEYIS™ may vary. Therefore, prescribers should evaluate the patient's response to KEVEYIS™ after 2 months of treatment to decide whether KEVEYIS™ should be continued.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
KEVEYIS™ is contraindicated in the following circumstances:
- Hypersensitivity to dichlorphenamide or other sulfonamides [see Warnings and Precautions (5.1)]
- Concomitant use of KEVEYIS™ and high dose aspirin [see Warnings and Precautions (5.2)]
- Severe pulmonary disease, limiting compensation to metabolic acidosis caused by KEVEYIS™ [see Warnings and Precautions (5.4)]
- Hepatic insufficiency: KEVEYIS™ may aggravate hepatic encephalopathy.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity / Anaphylaxis / Idiosyncratic Reactions
Fatalities associated with the administration of sulfonamides have occurred due to adverse reactions including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia and other blood dyscrasias. Pulmonary involvement can occur in isolation or as part of a systemic reaction.
KEVEYIS™ should be discontinued at the first appearance of skin rash or any sign of immune-mediated or idiosyncratic adverse reaction.
5.2 Concomitant Use of Aspirin
Anorexia, tachypnea, lethargy, and coma have been reported with concomitant use of dichlorphenamide and high-dose aspirin. The concomitant use of KEVEYIS™ and high dose aspirin is contraindicated. KEVEYIS™ should be used with caution in patients receiving low dose aspirin.
5.3 Hypokalemia
KEVEYIS™ increases potassium excretion and can cause hypokalemia. The risk of hypokalemia is greater when KEVEYIS™ is used in patients with conditions associated with hypokalemia (e.g., adrenocortical insufficiency, hyperchloremic metabolic acidosis, or respiratory acidosis), and in patients receiving other drugs that may cause hypokalemia (e.g., loop diuretics, thiazide diuretics, laxatives, antifungals, penicillin, and theophylline).
Baseline and periodic measurement of serum potassium during KEVEYIS™ treatment are recommended.
If hypokalemia develops or persists, consideration should be given to reducing the dose or discontinuing KEVEYIS™.
5.4 Metabolic Acidosis
KEVEYIS™ can cause hyperchloremic non-anion gap metabolic acidosis. Concomitant use of KEVEYIS™ with other drugs that cause metabolic acidosis may increase the severity of metabolic acidosis.
Baseline and periodic measurement of serum bicarbonate during KEVEYIS™ treatment are recommended.
If metabolic acidosis develops or persists, consideration should be given to reducing the dose or discontinuing KEVEYIS™.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in labeling:
- Hypersensitivity / Anaphylaxis / Idiosyncratic reactions [see Warnings and Precautions (5.1)]
- Hypokalemia [see Warnings and Precautions (5.3)]
- Metabolic Acidosis [see Warnings and Precautions (5.4)]
- Falls [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a 9-week randomized controlled trial in adults with hyperkalemic or hypokalemic periodic paralysis (Study 1), the most common adverse reactions in patients treated with KEVEYIS™, with rates greater than placebo, were paresthesia, cognitive disorder, dysgeusia, and confusional state. The mean dose of KEVEYIS™ was 94 mg/day in patients with hypokalemic periodic paralysis and 82 mg/day in patients with hyperkalemic periodic paralysis.
Table 1 lists the incidence of adverse reactions that occurred in ≥ 5% of patients treated with KEVEYIS™ and more commonly than in patients treated with placebo in Study 1.
Table 1: Adverse Reactions in Patients Treated with KEVEYIS™ with Incidence ≥ 5% and more common than in Patients Treated with Placebo in Study 1 Adverse Reaction KEVEYIS™
N = 36
(%)Placebo
N = 29
(%)- * Cognitive disorder combined cases with the preferred terms of cognitive disorder, disturbance in attention, and mental impairment.
Nervous system disorders Paresthesia 44 14 Cognitive disorder* 14 7 Dysgeusia 14 0 Confusional state 11 0 Headache 8 7 Hypoesthesia 8 0 Lethargy 8 0 Dizziness 6 0 Gastrointestinal disorders Diarrhea 6 3 Nausea 6 0 General disorders and administration site conditions Fatigue 8 0 Malaise 6 0 Investigations Weight decreased 6 0 Musculoskeletal and connective tissue disorders Muscle spasms 8 0 Arthralgia 6 3 Muscle twitching 6 0 Respiratory Dyspnea 6 0 Pharyngolaryngeal pain 6 0 Skin Rash 8 0 Pruritus 6 0 6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of dichlorphenamide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following are adverse reactions which have been reported for dichlorphenamide that were serious adverse events or are not reported in the previous section of labeling [see Clinical Trials Experience (6.1)]: amnesia, cardiac failure, condition aggravated, convulsion, fetal death, hallucination, nephrolithiasis, pancytopenia, psychotic disorder, renal tubular necrosis, stupor, syncope, tremor.
-
7 DRUG INTERACTIONS
7.1 Aspirin and Salicylates
KEVEYIS™ may cause an elevation in salicylate levels in patients receiving aspirin. Anorexia, tachypnea, lethargy, and coma have been reported with concomitant use of dichlorphenamide and high-dose aspirin.
Concomitant use of KEVEYIS™ and high dose aspirin is contraindicated. KEVEYIS™ should be used with caution in patients receiving low dose aspirin. [see Contraindications (4) and Warnings and Precautions (5.2)]
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C.
There are no adequate and well-controlled studies in pregnant women. Teratogenic effects (fetal limb reduction defects) were reported following oral administration of dichlorphenamide to pregnant rats during organogenesis at 350 mg/kg, or 17 times the maximum recommended human dose (200 mg/day) on a body surface area (mg/m2) basis. A no-effect dose has not been established. KEVEYIS™ should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
-
10 OVERDOSAGE
Symptoms of overdosage or toxicity may include drowsiness, anorexia, nausea, vomiting, dizziness, paresthesias, ataxia, tremor, and tinnitus.
In the event of overdosage, induce emesis or perform gastric lavage. The electrolyte disturbance most likely to be encountered from overdosage is hyperchloremic acidosis.
-
11 DESCRIPTION
KEVEYIS™ (dichlorphenamide) tablets is an oral carbonic anhydrase inhibitor. Dichlorphenamide, a dichlorinated benzenedisulfonamide, is known chemically as 4, 5–dichloro-1,3-benzenedisulfonamide.
Its empirical formula is C6H6Cl2N2O4S2 and its structural formula is:
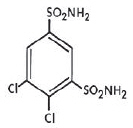
Dichlorphenamide USP is a white or practically white, crystalline compound with a molecular weight of 305.16. It is very slightly soluble in water but soluble in dilute solutions of sodium carbonate and sodium hydroxide. Dilute alkaline solutions of dichlorphenamide are stable at room temperature.
KEVEYIS™ (dichlorphenamide) tablets is supplied as tablets, for oral administration, each containing 50 mg dichlorphenamide. Inactive ingredients are lactose monohydrate, magnesium stearate and pregelatinized maize starch.
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The efficacy of KEVEYIS™ was evaluated in two clinical studies, Study 1 and Study 2.
Study 1
Study 1 was a 9-week, double blind, placebo-controlled multi-center study. Study 1 consisted of two substudies: a substudy in patients with hypokalemic periodic paralysis (n=44), and a substudy in patients with hyperkalemic periodic paralysis (n=21). The primary efficacy endpoint in both substudies was the average number of self-reported attacks of muscle weakness per week over the final 8 weeks of the trial. Withdrawal from the study for acute severe worsening was also assessed as an endpoint.
In Study 1, the dose of KEVEYIS™ was 50 mg b.i.d. for treatment-naïve patients. Patients already on dichlorphenamide prior to the study continued on the same dose while on KEVEYIS™ during the study. In patients taking acetazolamide prior to the study, the dose of KEVEYIS™ was set at 20% of the acetazolamide dose. Dose reduction for tolerability was permitted.
Hypokalemic Periodic Paralysis Substudy of Study 1
In the hypokalemic periodic paralysis substudy, median age of patients was 45 years and 73% of patients were male. Patients treated with KEVEYIS™ (n=24) had 2.2 fewer attacks per week than patients (n=20) treated with placebo (p=0.02). None of the patients randomized to KEVEYIS™ reached the endpoint of acute worsening, vs. five patients randomized to placebo. The mean dose of KEVEYIS™ at Week 9 was 94 mg/day.
Hyperkalemic Periodic Paralysis Substudy of Study 1
In the Hyperkalemic Periodic Paralysis substudy, median age of patients was 43 years and 43% of patients were male. During the double-blind treatment period, patients treated with KEVEYIS™ (n=12) had 3.9 fewer attacks per week than patients (n=9) treated with placebo (p=0.08). None of the patients randomized to KEVEYIS™ reached the endpoint of acute worsening, vs. two patients randomized to placebo. The mean dose of KEVEYIS™ at Week 9 was 82 mg/day.
Study 2
Study 2 was a 35-week, double blind, placebo-controlled, multi-center, two-period crossover study. Study 2 also consisted of two substudies: a substudy in a substudy in patients with hypokalemic periodic paralysis (n=42), and a substudy in patients with hyperkalemic periodic paralysis (n=31), including patients with Paramyotonia Congenita. The primary endpoint in the hypokalemic periodic paralysis substudy was the incidence of acute intolerable worsening (based on attack frequency or severity) necessitating withdrawal. The primary endpoint in the hyperkalemic periodic paralysis substudy was the average number of self-reported attacks of muscle weakness per week. Dosing was determined similarly to Study 1.
Hypokalemic Periodic Paralysis Substudy of Study 2
In the hypokalemic periodic paralysis substudy, mean age of patients was 38 years and 79% of patients were male. Acute intolerable worsening was observed in 2 patients on KEVEYIS™ vs. 11 patients on placebo (p=0.02). The mean dose of KEVEYIS™ at the end of the study was 96 mg/day.
Hyperkalemic Periodic Paralysis Substudy of Study 2
In the hyperkalemic periodic paralysis substudy, mean age of patients was 37 years and 79% of patients were male. Patients treated had 2.3 fewer attacks per week on KEVEYIS™ than on placebo (p=0.006). The mean dose of KEVEYIS™ at the end of the study was 73 mg/day.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Each KEVEYIS™ (dichlorphenamide) tablets, 50 mg – round, white tablet, scored on one side, engraved with "TARO" on one side and on the other side "D" above the score and "50" below the score.
KEVEYIS™ (dichlorphenamide) tablets are supplied as follows:
Bottles of 100 NDC: 51672-4177-1 - 17 PATIENT COUNSELING INFORMATION
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 50 mg Tablet Bottle Label
NDC: 51672-4177-1
100 TabletsKeveyis™
(dichlorphenamide)
Tablets 50 mgKeep this and all medications out
of the reach of children.
Rx only
-
INGREDIENTS AND APPEARANCE
KEVEYIS
dichlorphenamide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51672-4177 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dichlorphenamide (UNII: VVJ6673MHY) (Dichlorphenamide - UNII:VVJ6673MHY) Dichlorphenamide 50 mg Inactive Ingredients Ingredient Name Strength lactose monohydrate (UNII: EWQ57Q8I5X) magnesium stearate (UNII: 70097M6I30) starch, corn (UNII: O8232NY3SJ) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 6mm Flavor Imprint Code TARO;D;50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51672-4177-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 08/07/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA011366 08/07/2015 Labeler - Taro Pharmaceuticals U.S.A., inc. (145186370) Establishment Name Address ID/FEI Business Operations Taro Pharmaceutical Industries, Ltd. 600072078 MANUFACTURE(51672-4177)
Trademark Results [Keveyis]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KEVEYIS 86724075 5034655 Live/Registered |
TARO PHARMACEUTICALS U.S.A., INC. 2015-08-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.