Aloquin by Novum Pharma, LLC ALOQUIN Gel
Aloquin by
Drug Labeling and Warnings
Aloquin by is a Prescription medication manufactured, distributed, or labeled by Novum Pharma, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ALOQUIN- iodoquinol and aloe vera leaf gel
Novum Pharma, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
ALOQUIN Gel
DESCRIPTION
Each gram of ALOQUIN contains 1.25% (12.5 mg) Iodoquinol and 1% (10mg) Aloe Polysaccharides. Other ingredients: Purified Water, Carbomer 980, Magnesium Aluminum Silicate, PEG-20 Methyl Glucose Ether, Aminomethyl Propanol 95, Biopeptide, Propylene Glycol, Glycerine, SDA Alcohol 40 B, Benzyl Alcohol, Trolamine, FD&C Blue #1 and D&C Yellow #10.
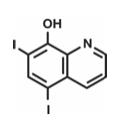
Iodoquinol
Iodoquinol is an antifungal and antibacterial agent. Chemically, Iodoquinol is [5,7-diiodo-8-quinolinol] with the molecular formula (C9H5I2NO) and is represented by the following structural formula:
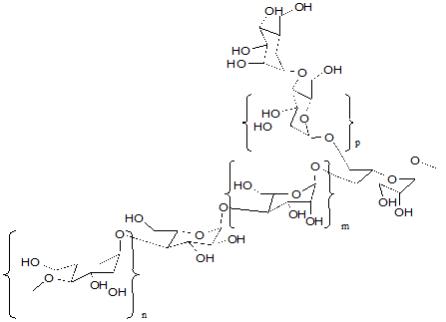
Aloe Polysaccharide
The Aloe Polysaccharide in ALOQUIN is a patented mixture of acetylated mannan aloe polysaccharide mixture with average molecular weights of 80 and 1300 kDa (CAS 89191-46-8). Each purified acetylated mannan polysaccharide of specific molecular weight range and average is composed of the same repeating subunits shown below (where m is mannose, n is galactose and p is glucose monomers):
INDICATIONS AND USAGE
Based on a review of a related drug by the National Research Council and subsequent FDA classification for that drug, the indications are as follows: “Possibly” Effective: Contact or atopic dermatitis; impetiginized eczema; nummular eczema; endogenous chronic infectious dermatitis; stasis dermatitis; pyoderma; nuchal eczema and chronic eczematoid otitis externa; acne urticata; localized or disseminated neurodermatitis; lichen simplex chronicus; anogenital pruritus (vulvae, scroti, ani); folliculitis; bacterial dermatoses; mycotic dermatoses such as tinea (capitis, cruris, corporis, pedis); monliasis; intertrigo. Final classification of the less-than-effective indications requires further investigation.
CONTRAINDICATIONS
ALOQUIN is contraindicated in those patients with a history of hypersensitivity to any components of the preparation.
WARNINGS AND PRECAUTIONS
For external use only. Keep away from eyes. If irritation develops, the use of ALOQUIN should be discontinued and appropriate therapy instituted. Some discoloration of the skin, hair and fabrics may occur, but can be removed with normal cleansing and laundry. Not intended for use on infants or under diapers or occlusive dressings.
Iodoquinol may be absorbed through the skin and interfere with thyroid function tests. If such tests are contemplated, wait at least one month after discontinuance of therapy to perform these tests. The ferric chloride test for phenylketonuria (PKU) can yield a false positive result if Iodoquinol is present in the diaper or urine. Prolonged use may result in overgrowth of non-susceptible organisms requiring appropriate therapy. Keep out of reach of children.
Carcinogenesis, Mutagenisis and Impairment of Fertility:
Long term animal studies have not been performed to evaluate the carcinogenic potential of the effect on fertility of Iodoquinol. Mutagenicity studies have not been performed with Iodoquinol.
Pregnancy Category C:
Animal reproductive studies have not been conducted with ALOQUIN. It is not known whether ALOQUIN can cause fetal harm when administered to pregnant women or can affect reproductive capacity. ALOQUIN should be given to pregnant women only if clearly needed.
ADVERSE REACTIONS
Adverse reactions from topical use of ALOQUIN is expected to be low when used as directed, due to low concentration of Iodoquinol present in this topical gel.
To achieve the equivalent of a common daily oral dose of nearly 2,000 mg Iodoquinol, one will need to use more than 2 full tubes of 60 g ALOQUIN in a single application. Adverse reactions from oral form of Iodoquinol (nearly 2,000 mg daily) have been reported: various forms of skin eruptions, hives, itching, nausea, vomiting, abdominal cramps, diarrhea, anusitis, fever, chills, headache, vertigo and enlargement of thyroid.
DOSAGE AND ADMINISTRATION
Apply to affected areas 3-4 times daily or as directed by a physician. Follow your physician’s directions regarding length of treatment after symptoms resolve.
HOW SUPPLIED
NDC # 69646-706-16……60 gram gel tube
NDC # 69646-706-01……1 gram gel individual pack
NDC # 69646-706-08……10-count carton of 1 gram gel sample packs – not for resale
Each 1 gram gel pack contains multiple doses depending on the surface area treated.
STORAGE
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (between 59°F and 86°F). Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimized.
Distributed by:
Novum Pharma, LLC
Chicago, IL 60654
NOVUM Logo
©2017 Novum Pharma, LLC. All rights reserved. 15905-0217
| ALOQUIN
iodoquinol and aloe vera leaf gel |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Novum Pharma, LLC (079736743) |