ALGENIST- octinoxate, titanium dioxide, zinc oxide cream
Algenist by
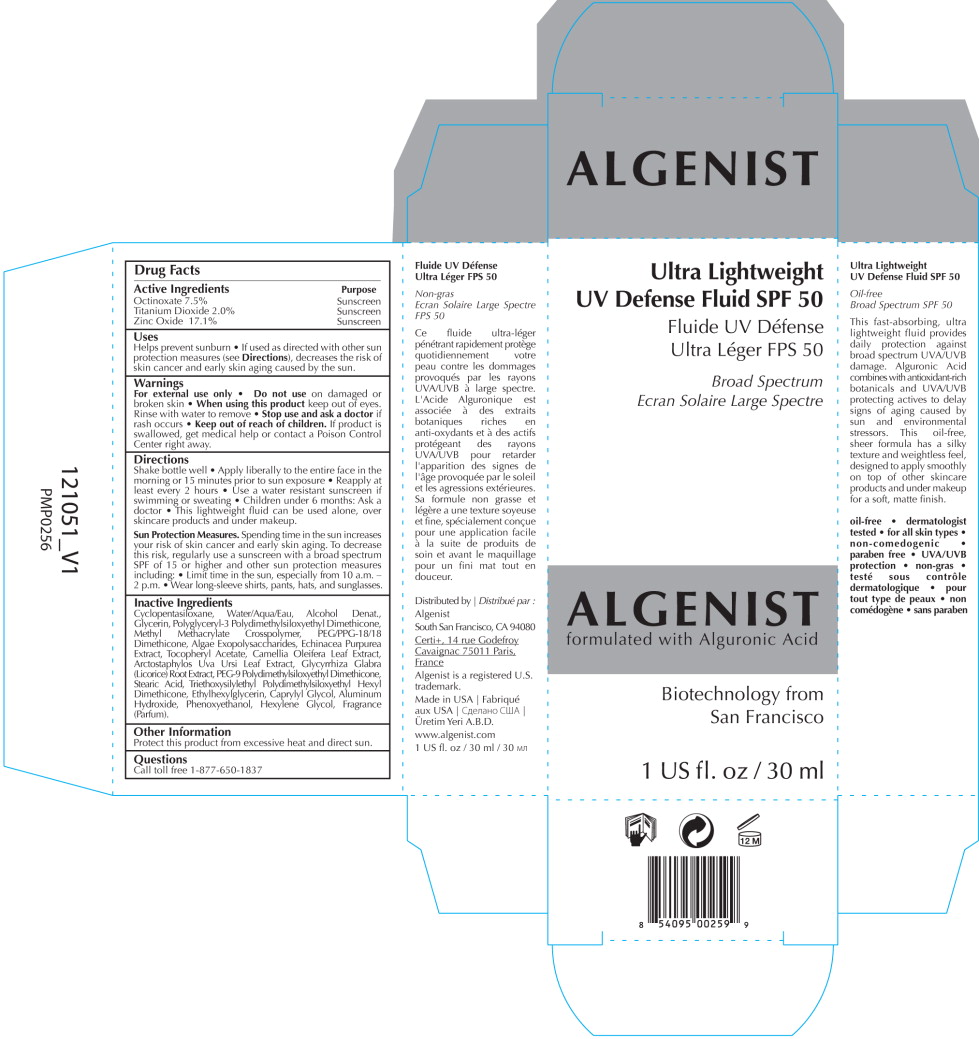
Drug Labeling and Warnings
Algenist by is a Otc medication manufactured, distributed, or labeled by Solazyme, Inc., PhytogenX,Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
-
Uses
Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
Shake bottle well
- Apply liberally to the entire face in the morning or 15 minutes prior to sun exposure
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Children under 6 months: Ask a doctor
- This lightweight fluid can be used alone, over skincare products and under makeup.
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeve shirts, pants, hats, and sunglasses.
-
Inactive Ingredients
Cyclopentasiloxane, Water/Aqua/Eau, Alcohol Denat., Glycerin, Polyglyceryl-3 Polydimethylsiloxyethyl Dimethicone, Methyl Methacrylate Crosspolymer, PEG/PPG-18/18 Dimethicone, Algae Exopolysaccharides, Echinacea Purpurea Extract, Tocopheryl Acetate, Camellia Oleifera Leaf Extract, Arctostaphylos Uva Ursi Leaf Extract, Glycyrrhiza Glabra (Licorice) Root Extract, PEG-9 Polydimethylsiloxyethyl Dimethicone, Stearic Acid, Triethoxysilylethyl Polydimethylsiloxyethyl Hexyl Dimethicone, Ethylhexylglycerin, Caprylyl Glycol, Aluminum Hydroxide, Phenoxyethanol, Hexylene Glycol, Fragrance (Parfum).
- Other Information
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALGENIST
octinoxate, titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 53407-138 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 75 mg in 1 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 20 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 171 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) water (UNII: 059QF0KO0R) alcohol (UNII: 3K9958V90M) glycerin (UNII: PDC6A3C0OX) METHYL METHACRYLATE (UNII: 196OC77688) peg/ppg-18/18 dimethicone (UNII: 9H0AO7T794) echinacea purpurea (UNII: QI7G114Y98) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) camellia oleifera seed (UNII: 59ED29FM2J) ARCTOSTAPHYLOS UVA-URSI LEAF (UNII: 3M5V3D1X36) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) stearic acid (UNII: 4ELV7Z65AP) ethylhexylglycerin (UNII: 147D247K3P) caprylyl glycol (UNII: 00YIU5438U) aluminum hydroxide (UNII: 5QB0T2IUN0) phenoxyethanol (UNII: HIE492ZZ3T) hexylene glycol (UNII: KEH0A3F75J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 53407-138-30 1 in 1 BOX 1 1 in 1 CARTON 1 30 mL in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 08/01/2012 Labeler - Solazyme, Inc. (145862012) Registrant - PhytogenX,Inc (010297942) Establishment Name Address ID/FEI Business Operations PhytogenX,Inc 010297942 MANUFACTURE(53407-138)
Trademark Results [Algenist]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALGENIST 85086990 3959874 Live/Registered |
Solazyme, Inc. 2010-07-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.