Eye Irrigating by Rugby Laboratories / Geri-Care Pharmaceuticals, Corp Rugby Eye Wash
Eye Irrigating by
Drug Labeling and Warnings
Eye Irrigating by is a Otc medication manufactured, distributed, or labeled by Rugby Laboratories, Geri-Care Pharmaceuticals, Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
EYE IRRIGATING- purified water solution
Rugby Laboratories
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Rugby Eye Wash
Uses
washes the eye to help relieve
- irritation
- stinging
- discomfort
- itching
by removing
- loose foreign material
- air pollutants (smog or pollen)
- chlorinated water
Warnings
For external use only
When using this product
- remove contact lenses before using
- do not touch tip of container to any surface to avoid contamination
- replace cap after use
Directions
For use without the eye cup
- flush the affected eye(s) as needed
- control the rate of flow of solution by placing pressure on the bottle

------------------------------------------------------------------------------
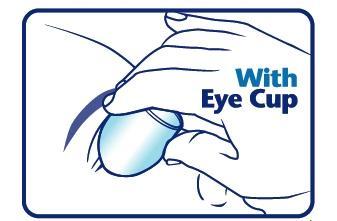
When using an eye cup
- rinse the cup with Eye Irrigating Solution immediately before each use
- avoid contamination of the rim and inside surfaces of the cup
- fill the cup half full with Eye Irrigating Solution and apply the cup to the affected eye(s), pressing tightly to prevent the escape of the liquid
- tilt the head backward. Open eyelids wide and rotate eyeball to thoroughly wash the eye
- rinse the cup with clean water after each use
Inactive ingredients
boric acid, sodium borate, sodium chloride, hydrochloric acid PRESERVATIVE ADDED: edetate disodium, polyhexamethylene biguanide
Package label
Rugby
NDC: 0536-1126-97
Compare to the active ingredient in Bausch + Lomb Advanced Eye Relief® Eye Wash*
GENTLE CLEANSING SOLUTION
FOR EVERYDAY USE
Eye
Irrigating
Solution
Eases Irritation
Soothes Burning & Stinging
Relieves Itching
THIMEROSAL FREE
Isotonic
Sterile eye cup enclosed
STERILE
4 FL OZ (118 mL)

| EYE IRRIGATING
purified water solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Rugby Laboratories (079246066) |
| Registrant - Geri-Care Pharmaceuticals, Corp (611196254) |