TENDERA-OB- ascorbic acid, cholecalciferol, .alpha.-tocopherol, thiamine mononitrate, riboflavin, niacin, pyridoxine hydrochloride, levomefolate glucosamine, cyanocobalamin, iron pentacarbonyl, potassium iodide, magnesium oxide, zinc gluconate, cupric oxide, and doconexent capsule
Tendera-OB by
Drug Labeling and Warnings
Tendera-OB by is a Other medication manufactured, distributed, or labeled by BonGeo Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
STATEMENT OF IDENTITY
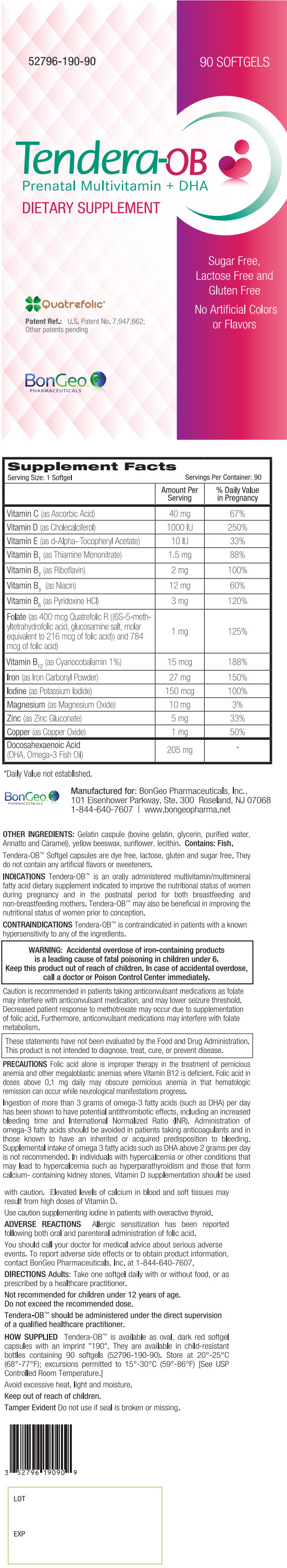
Supplement Facts Serving Size: 1 Softgel Servings Per Container: 90 Amount Per Serving % Daily Value in Pregnancy - * Daily Value not established.
Vitamin C (as Ascorbic Acid) 40 mg 67% Vitamin D (as Cholecalciferol) 1000 IU 250% Vitamin E (as d-Alpha- Tocopheryl Acetate) 10 IU 33% Vitamin B1 (as Thiamine Mononitrate) 1.5 mg 88% Vitamin B2 (as Riboflavin) 2 mg 100% Vitamin B3 (as Niacin) 12 mg 60% Vitamin B6 (as Pyridoxine HCl) 3 mg 120% Folate (as 400 mcg Quatrefolic R ((6S-5-meth-yltetrahydrofolic acid, glucosamine salt, molar equivalent to 216 mcg of folic acid)) and 784 mcg of folic acid) 1 mg 125% Vitamin B12 (as Cyanocobalamin 1%) 15 mcg 188% Iron (as Iron Carbonyl Powder) 27 mg 150% Iodine (as Potassium Iodide) 150 mcg 100% Magnesium (as Magnesium Oxide) 10 mg 3% Zinc (as Zinc Gluconate) 5 mg 33% Copper (as Copper Oxide) 1 mg 50% Docosahexaenoic Acid (DHA, Omega-3 Fish Oil) 205 mg * OTHER INGREDIENTS: Gelatin capsule (bovine gelatin, glycerin, purified water, Annatto and Caramel), yellow beeswax, sunflower, lecithin. Contains: Fish.
Tendera-OB™ Softgel capsules are dye free, lactose, gluten and sugar free. They do not contain any artificial flavors or sweeteners.
-
INDICATIONS
Tendera-OB™ is an orally administered multivitamin/multimineral fatty acid dietary supplement indicated to improve the nutritional status of women during pregnancy and in the postnatal period for both breastfeeding and non-breastfeeding mothers. Tendera-OB™ may also be beneficial in improving the nutritional status of women prior to conception.
- CONTRAINDICATIONS
- WARNINGS
-
HEALTH CLAIM
Caution is recommended in patients taking anticonvulsant medications as folate may interfere with anticonvulsant medication, and may lower seizure threshold. Decreased patient response to methotrexate may occur due to supplementation of folic acid. Furthermore, anticonvulsant medications may interfere with folate metabolism.
- HEALTH CLAIM
-
PRECAUTIONS
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and International Normalized Ratio (INR). Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding. Supplemental intake of omega 3 fatty acids such as DHA above 2 grams per day is not recommended. In individuals with hypercalcemia or other conditions that may lead to hypercalcemia such as hyperparathyroidism and those that form calcium- containing kidney stones, Vitamin D supplementation should be used with caution. Elevated levels of calcium in blood and soft tissues may result from high doses of Vitamin D.
Use caution supplementing iodine in patients with overactive thyroid.
-
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
You should call your doctor for medical advice about serious adverse events. To report adverse side effects or to obtain product information, contact BonGeo Pharmaceuticals, Inc. at 1-844-640-7607.
- DIRECTIONS
-
HOW SUPPLIED
Tendera-OB™ is available as oval, dark red softgel capsules with an imprint "190". They are available in child-resistant bottles containing 90 softgels (52796-190-90). Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature.]
Avoid excessive heat, light and moisture.
- SAFE HANDLING WARNING
- Tamper Evident
- HEALTH CLAIM
- HEALTH CLAIM
- PRINCIPAL DISPLAY PANEL - 90 Softgel Bottle Label
-
INGREDIENTS AND APPEARANCE
TENDERA-OB
ascorbic acid, cholecalciferol, .alpha.-tocopherol, thiamine mononitrate, riboflavin, niacin, pyridoxine hydrochloride, levomefolate glucosamine, cyanocobalamin, iron pentacarbonyl, potassium iodide, magnesium oxide, zinc gluconate, cupric oxide, and doconexent capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:52796-190 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 40 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 1000 [iU] .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) (.ALPHA.-TOCOPHEROL - UNII:H4N855PNZ1) .ALPHA.-TOCOPHEROL 10 [iU] THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 1.5 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 2 mg NIACIN (UNII: 2679MF687A) (NIACIN - UNII:2679MF687A) NIACIN 12 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 3 mg LEVOMEFOLATE GLUCOSAMINE (UNII: Q65PL71Q1A) (LEVOMEFOLATE GLUCOSAMINE - UNII:Q65PL71Q1A) LEVOMEFOLATE GLUCOSAMINE 1 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 15 ug IRON PENTACARBONYL (UNII: 6WQ62TAQ6Z) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 27 mg POTASSIUM IODIDE (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) POTASSIUM IODIDE 150 ug MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 10 mg ZINC GLUCONATE (UNII: U6WSN5SQ1Z) (ZINC CATION - UNII:13S1S8SF37) ZINC GLUCONATE 5 mg CUPRIC OXIDE (UNII: V1XJQ704R4) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 1 mg DOCONEXENT (UNII: ZAD9OKH9JC) (DOCONEXENT - UNII:ZAD9OKH9JC) DOCONEXENT 205 mg Inactive Ingredients Ingredient Name Strength glycerin (UNII: PDC6A3C0OX) water (UNII: 059QF0KO0R) annatto (UNII: 6PQP1V1B6O) caramel (UNII: T9D99G2B1R) yellow wax (UNII: 2ZA36H0S2V) helianthus annuus whole (UNII: 17S27ZT6KR) fish, unspecified (UNII: 1PIO77PW2X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:52796-190-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 03/19/2019 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 15 mm imprint Labeler - BonGeo Pharmaceuticals, Inc. (964822022)
Trademark Results [Tendera-OB]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TENDERA-OB 88251976 not registered Live/Pending |
BonGeo Pharmaceuticals Inc. 2019-01-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.