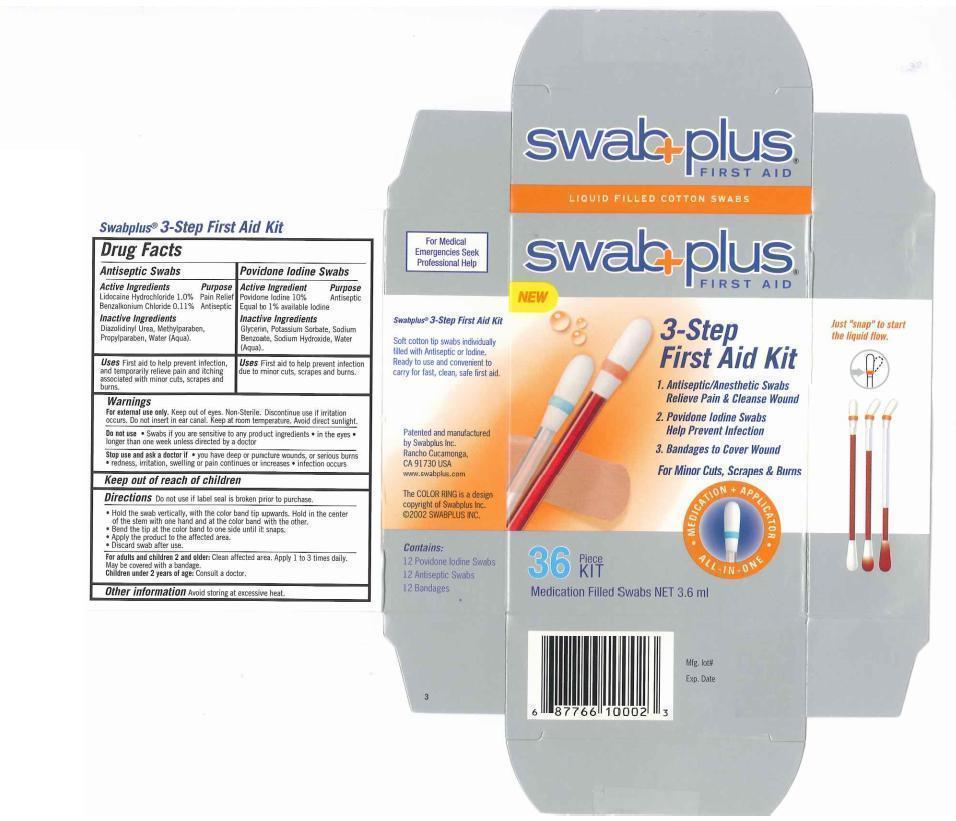
3-STEP FIRST AID KIT- povidone-iodine, lidocaine hydrochloride, benzalkonium chloride kit
3-Step First Aid Kit by
Drug Labeling and Warnings
3-Step First Aid Kit by is a Otc medication manufactured, distributed, or labeled by Swabplus Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug FactsActive Ingredients
- Purpose
- Uses
- Keep out of reach of children
-
Directions
- Do not use if label seal is broken prior to purchase.
- Hold the swab vertically, with the color ring band tip upwards. Hold in the center of the stem with one hand and at the color band with the other.
- Bend the tip at the color band to one side until it snaps.
- Apply the product to the affected area.
- Discard swab after use.
-
Warnings
For external use only. Keep out of eyes. Non-Sterile. Discontinue use if irritation occurs. Do not insert inear canal. Keep at room temperture. Avoid direct sunlight.
Do not use. Swabs if you are sensitive to any product ingredients. in the eyes. longer than one week unless directed by a doctor.
Stop use and ask a doctor if you have deep or punchture wounds, or serious burns. redness, irritation, sweeling or pain continues or increases, infection occurs
- Other information
- Inactive Ingredients
- Manufacturer Statement
- Package and label
-
INGREDIENTS AND APPEARANCE
3-STEP FIRST AID KIT
povidone-iodine, lidocaine hydrochloride, benzalkonium chloride kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 65734-000 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65734-000-23 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 2 mL Part 2 1 PACKET 2 mL Part 1 of 2 ANTISEPTIC AND ANESTHETIC SWAB
lidocaine hydrochloride, benzalkonium chloride liquidProduct Information Item Code (Source) NDC: 65734-125 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 100 mg in 10 mL Benzalkonium Chloride (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) Benzalkonium Chloride 11 mg in 10 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Diazolidinyl Urea (UNII: H5RIZ3MPW4) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Propylene Glycol (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65734-125-01 2 mL in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part348 03/01/2003 Part 2 of 2 POVIDONE-IODINE SWAB
povidone-iodine liquidProduct Information Item Code (Source) NDC: 65734-119 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Povidone-Iodine (UNII: 85H0HZU99M) (Iodine - UNII:9679TC07X4) Iodine 100 mg in 10 mL Inactive Ingredients Ingredient Name Strength Sodium Hydroxide (UNII: 55X04QC32I) Glycerin (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65734-119-01 2 mL in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 03/01/2003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 03/01/2003 Labeler - Swabplus Inc. (876441549) Registrant - Swabplus Inc. (876441549) Establishment Name Address ID/FEI Business Operations Swabplus Inc. 876441549 repack(65734-000) , manufacture(65734-125, 65734-119)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.