Enteric Coated Aspirin by CARDINAL HEALTH / TIME CAP LABORATORIES, INC LEADER 331R
Enteric Coated Aspirin by
Drug Labeling and Warnings
Enteric Coated Aspirin by is a Otc medication manufactured, distributed, or labeled by CARDINAL HEALTH, TIME CAP LABORATORIES, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
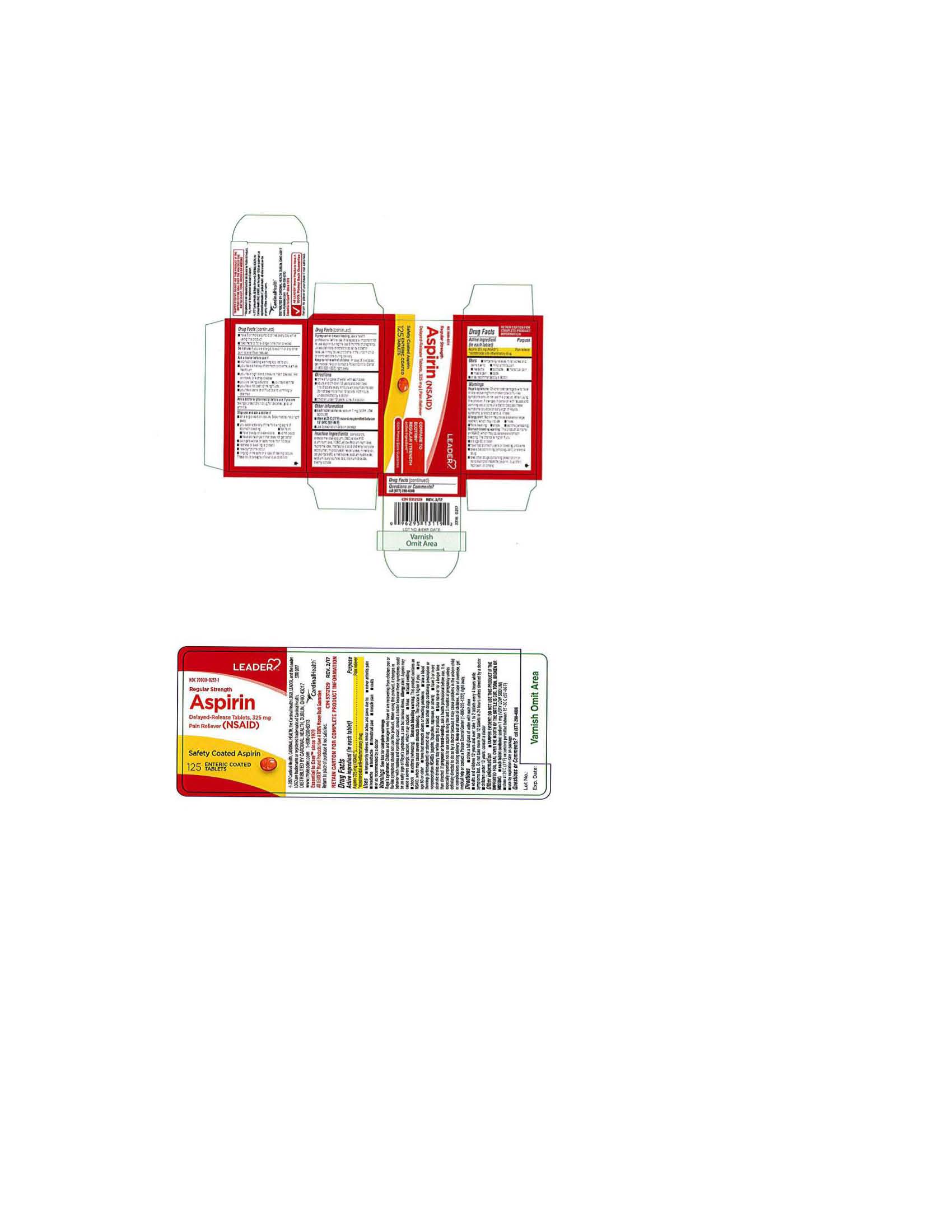
ENTERIC COATED ASPIRIN REGULAR STRENGTH- aspirin tablet, delayed release
CARDINAL HEALTH
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
LEADER 331R
| Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center (1-800 222-1222) right away |
|
Directions
|
| corn starch, croscarmellose sodium,D-C yellow #10 aluminum lake, FD-C yellow#6 aluminum lake, hypromellose, methacrylic acid copolymer, microcrystalline cellulose,mineral oil, polysorbate 80, simethicone, sodium hydroxide, sodium lauryl sulfate, talc, titanium dioxide, triethyl citrate |
|
Use for the temporary relief of minor aches and pains due to: headache, colds, muscle pain, menstrual pain, toothache, minor pain of arthritis or as directed by your doctor |
|
Warnings: Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness. Allergy alert: Aspirin may cause a severe allergic reaction, which may include: hives, facial swelling, shock, asthmaaa9wheezing) Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you are age 60 or older, have had stomach ulcers or bleeding problems, take a blood thinning (anticoagulant) or steroid drug, take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen or others), have 3 or more alcoholic drinks every day while using this product, take more or for a longer time than directed. |
| ENTERIC COATED ASPIRIN
REGULAR STRENGTH
aspirin tablet, delayed release |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - CARDINAL HEALTH (097537435) |
| Registrant - TIME CAP LABORATORIES, INC (037052099) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TIME CAP LABORATORIES, INC | 037052099 | manufacture(70000-0237) | |