ACCURATE LABS ANTISEPTIC HAND SANITIZER
ACCURATE LABS ANTISEPTIC HAND SANITIZER by
Drug Labeling and Warnings
ACCURATE LABS ANTISEPTIC HAND SANITIZER by is a Otc medication manufactured, distributed, or labeled by Accurate Labs, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ACCURATE LABS ANTISEPTIC HAND SANITIZER- ethyl alcohol gel
Accurate Labs, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
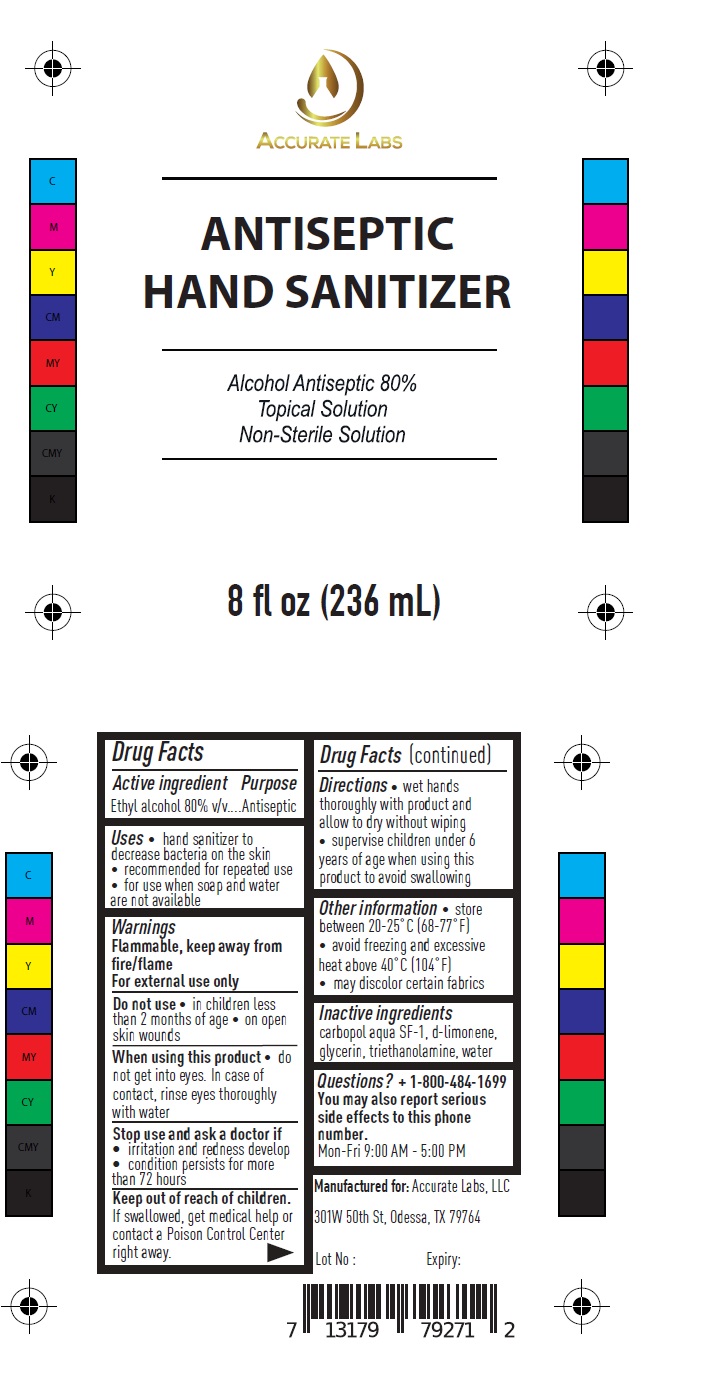
ACCURATE LABS ANTISEPTIC HAND SANITIZER
Uses
hand sanitizer to decrease bacteria on the skin
recommended for repeated use
for use when soap and water are not available
Warnings
Flammable, keep away from fire/flame
For external use only
Do not use in children less than 2 months of age on open skin wounds
When using this product do not get into eyes. In case of contact, rinse eyes thoroughly with water
Stop use and ask a doctor if
irritation and redness develop
condition persists for more than 72 hours
Directions
- wet hands thoroughly with product and allow to dry without wiping
- supervise children under 6 years of age when using this product to avoid swallowing
Other information
store between 20-25˚C (68-77˚F)
avoid freezing and excessive heat above 40˚C (104˚F)
may discolor certain fabrics
Questions?
1-800-484-1699
You may also report serious side effects to this phone number.
Mon-Fri 9:00 AM - 5:00 PM
| ACCURATE LABS ANTISEPTIC HAND SANITIZER
ethyl alcohol gel |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Accurate Labs, LLC (113427259) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Accurate Labs, LLC | 113427259 | manufacture(78434-100) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.