CAMPATH- alemtuzumab injection
Campath by
Drug Labeling and Warnings
Campath by is a Prescription medication manufactured, distributed, or labeled by Genzyme Corporation, Boehringer Ingelheim Pharma GmbH & Co. KG (BI Pharma), Sanofi-Aventis Deutschland GmbH, Genzyme Limited, Genzyme Ireland Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CAMPATH safely and effectively. See full prescribing information for CAMPATH.
CAMPATH® (alemtuzumab) injection, for intravenous use
Initial U.S. Approval: 2001WARNING: CYTOPENIAS, INFUSION REACTIONS, AND INFECTIONS
See full prescribing information for complete boxed warning.
Serious, including fatal, cytopenias, infusion reactions, and infections can occur (5.1– 5.3).
- Limit doses to 30 mg (single) and 90 mg (cumulative weekly); higher doses increase risk of pancytopenia. (2.1)
- Escalate dose gradually and monitor patients during infusion. Withhold therapy for Grade 3 or 4 infusion reactions. (5.2)
- Administer prophylaxis against Pneumocystis jirovecii pneumonia (PCP) and herpes virus infections. (2.2, 5.3)
INDICATIONS AND USAGE
CAMPATH is a CD52-directed cytolytic antibody indicated as a single agent for the treatment of B-cell chronic lymphocytic leukemia (B-CLL). (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injection: 30 mg/1 mL single dose vial (3).
CONTRAINDICATIONS
None (4).
WARNINGS AND PRECAUTIONS
Cytopenias:
Obtain complete blood counts (CBC) and platelet counts at weekly intervals during therapy and CD4 counts after therapy until recovery to ≥200 cells/µL. (5.4) Discontinue for autoimmune or severe hematologic adverse reactions. (5.1)
Infections:
CAMPATH induces severe and prolonged lymphopenia and increases risk of infection. If a serious infection occurs, withhold treatment until infection resolves. (5.3) Do not administer live viral vaccines to patients who have recently received CAMPATH. (5.5)
ADVERSE REACTIONS
Most common adverse reactions (≥10%): cytopenias, infusion reactions, cytomegalovirus (CMV) and other infections, nausea, emesis, diarrhea, and insomnia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Genzyme Corporation at 1-877-4-CAMPATH (1-877-422-6728) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CYTOPENIAS, INFUSION REACTIONS, AND INFECTIONS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Schedule and Administration
2.2 Recommended Concomitant Medications
2.3 Dose Modification
2.4 Preparation and Administration
2.5 Incompatibilities
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cytopenias
5.2 Infusion Reactions
5.3 Immunosuppression/Infections
5.4 Laboratory Monitoring
5.5 Immunization
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Previously Untreated B-CLL Patients
14.2 Previously Treated B-CLL Patients
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CYTOPENIAS, INFUSION REACTIONS, AND INFECTIONS
Cytopenias: Serious, including fatal, pancytopenia/marrow hypoplasia, autoimmune idiopathic thrombocytopenia, and autoimmune hemolytic anemia can occur in patients receiving CAMPATH. Single doses of CAMPATH greater than 30 mg or cumulative doses greater than 90 mg per week increase the incidence of pancytopenia [see Warnings and Precautions (5.1)].
Infusion Reactions: CAMPATH administration can result in serious, including fatal, infusion reactions. Carefully monitor patients during infusions and withhold CAMPATH for Grade 3 or 4 infusion reactions. Gradually escalate CAMPATH to the recommended dose at the initiation of therapy and after interruption of therapy for 7 or more days [see Dosage and Administration (2) and Warnings and Precautions (5.2)].
Infections: Serious, including fatal, bacterial, viral, fungal, and protozoan infections can occur in patients receiving CAMPATH. Administer prophylaxis against Pneumocystis jirovecii pneumonia (PCP) and herpes virus infections [see Dosage and Administration (2.2) and Warnings and Precautions (5.3)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Schedule and Administration
- Administer as an IV infusion over 2 hours. Do not administer as intravenous push or bolus.
- Recommended Dosing Regimen
- Gradually escalate to the maximum recommended single dose of 30 mg. Escalation is required at initiation of dosing or if dosing is held ≥7 days during treatment. Escalation to 30 mg ordinarily can be accomplished in 3 to 7 days.
- Escalation Strategy:
- – Administer 3 mg daily until infusion reactions are ≤ grade 2 [see Adverse Reactions (6.1)].
- – Then administer 10 mg daily until infusion reactions are ≤ grade 2.
- – Then administer 30 mg/day three times per week on alternate days (e.g., Mon-Wed-Fri). The total duration of therapy, including dose escalation, is 12 weeks.
- Single doses of greater than 30 mg or cumulative doses greater than 90 mg per week increase the incidence of pancytopenia.
2.2 Recommended Concomitant Medications
- Premedicate with diphenhydramine (50 mg) and acetaminophen (500–1000 mg) 30 minutes prior to first infusion and each dose escalation. Institute appropriate medical management (e.g. steroids, epinephrine, meperidine) for infusion reactions as needed [see Boxed Warning, Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
- Administer trimethoprim/sulfamethoxazole DS twice daily (BID) three times per week (or equivalent) as Pneumocystis jirovecii pneumonia (PCP) prophylaxis.
- Administer famciclovir 250 mg BID or equivalent as herpetic prophylaxis.
Continue PCP and herpes viral prophylaxis for a minimum of 2 months after completion of CAMPATH or until the CD4+ count is ≥200 cells/µL, whichever occurs later [see Boxed Warning and Warnings and Precautions (5.3)].
2.3 Dose Modification
- Withhold CAMPATH during serious infection or other serious adverse reactions until resolution.
- Discontinue CAMPATH for autoimmune anemia or autoimmune thrombocytopenia.
- There are no dose modifications recommended for lymphopenia.
Dose Modification for Neutropenia or Thrombocytopenia [see Warnings and Precautions (5.1)] Hematologic Values Dose Modification* - * If the delay between dosing is ≥7 days, initiate therapy at CAMPATH 3 mg and escalate to 10 mg and then to 30 mg as tolerated [see Dosage and Administration (2.1)].
ANC <250/μL and/or platelet count ≤25,000/μL For first occurrence: Withhold CAMPATH therapy. Resume CAMPATH at 30 mg when ANC ≥500/μL and platelet count ≥50,000/μL. For second occurrence: Withhold CAMPATH therapy. Resume CAMPATH at 10 mg when ANC ≥500/μL and platelet count ≥50,000/μL. For third occurrence: Discontinue CAMPATH therapy. ≥50% decrease from baseline in patients initiating therapy with a baseline ANC ≤250/μL and/or a baseline platelet count ≤25,000/μL For first occurrence: Withhold CAMPATH therapy. Resume CAMPATH at 30 mg upon return to baseline value(s). For second occurrence: Withhold CAMPATH therapy. Resume CAMPATH at 10 mg upon return to baseline value(s). For third occurrence: Discontinue CAMPATH therapy. 2.4 Preparation and Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. If particulate matter is present or the solution is discolored, the vial should not be used. DO NOT SHAKE VIAL.
Use aseptic technique during the preparation and administration of CAMPATH. Withdraw the necessary amount of CAMPATH from the vial into a syringe.
- To prepare the 3 mg dose, withdraw 0.1 mL into a 1 mL syringe calibrated in increments of 0.01 mL.
- To prepare the 10 mg dose, withdraw 0.33 mL into a 1 mL syringe calibrated in increments of 0.01 mL.
- To prepare the 30 mg dose, withdraw 1 mL in either a 1 mL or 3 mL syringe calibrated in 0.1 mL increments.
Inject syringe contents into 100 mL sterile 0.9% Sodium Chloride USP or 5% Dextrose in Water USP. Gently invert the bag to mix the solution. Discard syringe.
The vial contains no preservatives and is intended for single use only. DISCARD VIAL including any unused portion after withdrawal of dose.
Use within 8 hours after dilution. Store diluted CAMPATH at room temperature (15°C–30°C) or refrigerated (2°C–8°C). Protect from light.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cytopenias
Severe, including fatal, autoimmune anemia and thrombocytopenia, and prolonged myelosuppression have been reported in patients receiving CAMPATH.
In addition, hemolytic anemia, pure red cell aplasia, bone marrow aplasia, and hypoplasia have been reported after treatment with CAMPATH at the recommended dose. Single doses of CAMPATH greater than 30 mg or cumulative doses greater than 90 mg per week increase the incidence of pancytopenia.
Withhold CAMPATH for severe cytopenias (except lymphopenia). Discontinue for autoimmune cytopenias or recurrent/persistent severe cytopenias (except lymphopenia) [see Dosage and Administration (2.3)]. No data exist on the safety of CAMPATH resumption in patients with autoimmune cytopenias or marrow aplasia [see Adverse Reactions (6.1)].
5.2 Infusion Reactions
Adverse reactions occurring during or shortly after CAMPATH infusion include pyrexia, chills/rigors, nausea, hypotension, urticaria, dyspnea, rash, emesis, and bronchospasm. In clinical trials, the frequency of infusion reactions was highest in the first week of treatment. Monitor for the signs and symptoms listed above and withhold infusion for Grade 3 or 4 infusion reactions [see Adverse Reactions (6.1)].
The following serious, including fatal, infusion reactions have been identified in postmarketing reports: syncope, pulmonary infiltrates, acute respiratory distress syndrome (ARDS), respiratory arrest, cardiac arrhythmias, myocardial infarction, acute cardiac insufficiency, cardiac arrest, angioedema, and anaphylactoid shock.
Initiate CAMPATH according to the recommended dose-escalation scheme [see Dosage and Administration (2)]. Premedicate patients with an antihistamine and acetaminophen prior to dosing. Institute medical management (e.g., glucocorticoids, epinephrine, meperidine) for infusion reactions as needed [see Dosage and Administration (2.2)]. If therapy is interrupted for 7 or more days, reinstitute CAMPATH with gradual dose escalation [see Dosage and Administration (2.3) and Adverse Reactions (6)].
5.3 Immunosuppression/Infections
CAMPATH treatment results in severe and prolonged lymphopenia with a concomitant increased incidence of opportunistic infections [see Adverse Reactions (6.1)]. Administer PCP and herpes viral prophylaxis during CAMPATH therapy and for a minimum of 2 months after completion of CAMPATH or until the CD4+ count is ≥200 cells/µL, whichever occurs later [see Dosage and Administration (2.2)]. Prophylaxis does not eliminate these infections.
Routinely monitor patients for CMV infection during CAMPATH treatment and for at least 2 months following completion of treatment. Withhold CAMPATH for serious infections and during antiviral treatment for CMV infection or confirmed CMV viremia (defined as polymerase chain reaction (PCR) positive CMV in ≥2 consecutive samples obtained 1 week apart) [see Adverse Reactions (6.1)]. Initiate therapeutic ganciclovir (or equivalent) for CMV infection or confirmed CMV viremia [see Dosage and Administration (2.3)].
Administer only irradiated blood products to avoid transfusion associated Graft versus Host Disease (TAGVHD), unless emergent circumstances dictate immediate transfusion.
In patients receiving CAMPATH as initial therapy, recovery of CD4+ counts to ≥200 cells/µL occurred by 6 months post treatment; however at 2 months post treatment, the median was 183 cells/µL. In previously treated patients receiving CAMPATH, the median time to recovery of CD4+ counts to ≥200 cells/µL was 2 months; however, full recovery (to baseline) of CD4+ and CD8+ counts may take more than 12 months [see Boxed Warning and Adverse Reactions (6)].
5.4 Laboratory Monitoring
Obtain complete blood counts (CBC) at weekly intervals during CAMPATH therapy and more frequently if worsening anemia, neutropenia, or thrombocytopenia occurs. Assess CD4+ counts after treatment until recovery to ≥200 cells/µL [see Dosage and Administration (5.3) and Adverse Reactions (6)].
5.5 Immunization
The safety of immunization with live viral vaccines following CAMPATH therapy has not been studied. Do not administer live viral vaccines to patients or infants born to patients receiving CAMPATH. The ability to generate an immune response to any vaccine following CAMPATH therapy has not been studied.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the label:
- Cytopenias [see Warnings and Precautions (5.1)]
- Infusion Reactions [see Warnings and Precautions (5.2)]
- Immunosuppression/Infections [see Warnings and Precautions (5.3)]
The most common adverse reactions with CAMPATH are: infusion reactions (pyrexia, chills, hypotension, urticaria, nausea, rash, tachycardia, dyspnea), cytopenias (neutropenia, lymphopenia, thrombocytopenia, anemia), infections (CMV viremia, CMV infection, other infections), gastrointestinal symptoms (nausea, emesis, abdominal pain), and neurological symptoms (insomnia, anxiety). The most common serious adverse reactions are cytopenias, infusion reactions, and immunosuppression/infections.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data below reflect exposure to CAMPATH in 296 patients with CLL of whom 147 were previously untreated and 149 received at least 2 prior chemotherapy regimens. The median duration of exposure was 11.7 weeks for previously untreated patients and 8 weeks for previously treated patients.
Lymphopenia
Severe lymphopenia and a rapid and sustained decrease in lymphocyte subsets occurred in previously untreated and previously treated patients following administration of CAMPATH. In previously untreated patients, the median CD4+ was 0 cells/μL at one month after treatment and 238 cells/μL [25%–75% interquartile range 115 to 418 cells/μL at 6 months post treatment [see Warnings and Precautions (5.3)].
Neutropenia
In previously untreated patients, the incidence of Grade 3 or 4 neutropenia was 42% with a median time to onset of 31 days and a median duration of 37 days. In previously treated patients, the incidence of Grade 3 or 4 neutropenia was 64% with a median duration of 28 days. Ten percent of previously untreated patients and 17% of previously treated patients received granulocyte colony stimulating factors.
Anemia
In previously untreated patients, the incidence of Grade 3 or 4 anemia was 12% with a median time to onset of 31 days and a median duration of 8 days. In previously treated patients, the incidence of Grade 3 or 4 anemia was 38%. Seventeen percent of previously untreated patients and 66% of previously treated patients received either erythropoiesis stimulating agents, transfusions or both.
Thrombocytopenia
In previously untreated patients, the incidence of Grade 3 or 4 thrombocytopenia was 14% with a median time to onset of 9 days and a median duration of 14 days. In previously treated patients, the incidence of Grade 3 or 4 thrombocytopenia was 52% with a median duration of 21 days. Autoimmune thrombocytopenia was reported in 2% of previously treated patients with one fatality.
Infusion Reactions
Infusion reactions, which included pyrexia, chills, hypotension, urticaria, and dyspnea, were common. Grade 3 and 4 pyrexia and/or chills occurred in approximately 10% of previously untreated patients and in approximately 35% of previously treated patients. The occurrence of infusion reactions was greatest during the initial week of treatment and decreased with subsequent doses of CAMPATH. All patients were pretreated with antipyretics and antihistamines; additionally, 43% of previously untreated patients received glucocorticoid pre-treatment.
Infections
In the study of previously untreated patients, patients were tested weekly for CMV using a PCR assay from initiation through completion of therapy, and every 2 weeks for the first 2 months following therapy. CMV infection occurred in 16% (23/147) of previously untreated patients; approximately one-third of these infections were serious or life threatening. In studies of previously treated patients in which routine CMV surveillance was not required, CMV infection was documented in 6% (9/149) of patients; nearly all of these infections were serious or life threatening.
Other infections were reported in approximately 50% of patients across all studies. Grade 3 to 5 sepsis ranged from 3% to 10% across studies and was higher in previously treated patients. Grade 3 to 4 febrile neutropenia ranged from 5% to 10% across studies and was higher in previously treated patients. Infection-related fatalities occurred in 2% of previously untreated patients and 16% of previously treated patients. There were 198 episodes of other infection in 109 previously untreated patients; 16% were bacterial, 7% were fungal, 4% were other viral, and in 73% the organism was not identified.
Cardiac
Cardiac dysrhythmias occurred in approximately 14% of previously untreated patients. The majority were tachycardias and were temporally associated with infusion; dysrhythmias were Grade 3 or 4 in 1% of patients.
Previously Untreated Patients
Table 1 contains selected adverse reactions observed in 294 patients randomized (1:1) to receive CAMPATH or chlorambucil as first line therapy for B-CLL. CAMPATH was administered at a dose of 30 mg intravenously three times weekly for up to 12 weeks. The median duration of therapy was 11.7 weeks with a median weekly dose of 82 mg (25–75% interquartile range: 69–90 mg).
Table 1: Per Patient Incidence of Selected* Adverse Reactions in Treatment Naive B-CLL Patients CAMPATH (n=147) Chlorambucil (n=147) All Grades†
%Grades 3–4
%All Grades
%Grades 3–4
%- * Adverse reactions occurring at a higher relative frequency in the CAMPATH arm
- † NCI CTC version 2.0 for adverse reactions; NCI CTCAE version 3.0 for laboratory values
- ‡ CMV viremia (without evidence of symptoms) includes both cases of single PCR positive test results and of confirmed CMV viremia (≥2 occasions in consecutive samples 1 week apart). For the latter, ganciclovir (or equivalent) was initiated per protocol.
Blood and Lymphatic System Disorders Lymphopenia 97 97 9 1 Neutropenia 77 42 51 26 Anemia 76 13 54 18 Thrombocytopenia 71 13 70 14 General Disorders and Administration Site Conditions Pyrexia 69 10 11 1 Chills 53 3 1 0 Infections and Infestations CMV viremia‡ 55 4 8 0 CMV infection 16 5 0 0 Other infections 74 21 65 10 Skin and Subcutaneous Tissue Disorders Urticaria 16 2 1 0 Rash 13 1 4 0 Erythema 4 0 1 0 Vascular Disorders Hypotension 16 1 0 0 Hypertension 14 5 2 1 Nervous System Disorders Headache 14 1 8 0 Tremor 3 0 1 0 Respiratory, Thoracic and Mediastinal Disorders Dyspnea 14 4 7 3 Gastrointestinal Disorders Diarrhea 10 1 4 0 Psychiatric Disorders Insomnia 10 0 3 0 Anxiety 8 0 1 0 Cardiac Disorders Tachycardia 10 0 1 0 Previously Treated Patients
Additional safety information was obtained from 3 single arm studies of 149 previously treated patients with CLL administered 30 mg CAMPATH intravenously three times weekly for 4 to 12 weeks (median cumulative dose 673 mg [range 2–1106 mg]; median duration of therapy 8.0 weeks). Adverse reactions in these studies not listed in Table 1 that occurred at an incidence rate of >5% were fatigue, nausea, emesis, musculoskeletal pain, anorexia, dysesthesia, mucositis, and bronchospasm.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. Using an ELISA assay, anti-human antibodies (HAHA) were detected in 11 of 133 (8.3%) previously untreated patients. In addition, two patients were weakly positive for neutralizing activity. Limited data suggest that the anti-CAMPATH antibodies did not adversely affect tumor response. Four of 211 (1.9%) previously treated patients were found to have antibodies to CAMPATH following treatment.
The incidence of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to CAMPATH with the incidence of antibodies to other products may be misleading.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post approval use of alemtuzumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Fatal infusion reactions
Cardiovascular: congestive heart failure, cardiomyopathy, decreased ejection fraction (some patients had been previously treated with cardiotoxic agents).
Cerebrovascular Disorders: Cervicocephalic arterial dissection, stroke, including hemorrhagic and ischemic stroke
Gastrointestinal: Acute acalculous cholecystitis
Immune disorders: Goodpasture's syndrome, Graves' disease, aplastic anemia, Guillain Barré syndrome, chronic inflammatory demyelinating polyradiculoneuropathy, serum sickness, fatal transfusion associated graft versus host disease.
Infections: Epstein-Barr virus (EBV) including EBV-associated lymphoproliferative disorder, progressive multifocal leukoencephalopathy (PML), reactivation of latent viruses.
Metabolic: tumor lysis syndrome.
Neurologic: optic neuropathy.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies, CAMPATH may cause fetal harm when administered to a pregnant woman.
Available data from published cohort studies in pregnant women are insufficient to establish a CAMPATH-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Alemtuzumab was embryolethal in pregnant huCD52 transgenic mice when administered during organogenesis (see Data). Human IgG antibodies are known to cross the placental barrier; therefore, CAMPATH may be transmitted from the mother to the developing fetus. Advise women of the potential risk to the fetus. Infants born to pregnant women treated with CAMPATH may be at increased risk of infection (see Clinical Considerations). The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal adverse reactions
Monoclonal antibodies are transported across the placenta as pregnancy progresses, with the largest amount transferred during the third trimester. Consider the risks and benefits of administering live or live-attenuated vaccines to infants exposed to CAMPATH in utero [see Warnings and Precautions (5.3, 5.5)].
Data
Animal Data
When alemtuzumab was administered to pregnant huCD52 transgenic mice during organogenesis (gestation days [GD] 6–10 or GD 11–15) at doses of 3 or 10 mg/kg IV, no teratogenic effects were observed. However, there was an increase in embryolethality (increased postimplantation loss and the number of dams with all fetuses dead or resorbed) in pregnant animals dosed during GD 11–15. In a separate study in pregnant huCD52 transgenic mice, administration of alemtuzumab during organogenesis (GD 6–10 or GD 11–15) at doses of 3 or 10 mg/kg IV, decreases in B-lymphocyte and T-lymphocyte populations were observed in the offspring at both doses tested.
In pregnant huCD52 transgenic mice administered alemtuzumab at doses of 3 or 10 mg/kg/day IV throughout gestation and lactation, there was an increase in pup deaths during the lactation period at 10 mg/kg. Decreases in T-lymphocyte and B-lymphocyte populations and in antibody response were observed in offspring at both doses tested.
8.2 Lactation
Risk Summary
There are no data on the presence of alemtuzumab in human milk, effects on milk production, or the breastfed child. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed infant to alemtuzumab are unknown. Alemtuzumab was detected in the milk of lactating huCD52 transgenic mice administered alemtuzumab (see Data). Maternal IgG is known to be present in human milk and when a drug is present in animal milk, it is likely that the drug will be present in human milk.
Because of the potential for serious adverse reactions from CAMPATH in a breastfed child, including reduced lymphocyte counts, advise lactating women not to breastfeed during treatment with CAMPATH and for at least 3 months following the last dose.
Data
Alemtuzumab was detected in the milk of lactating huCD52 transgenic mice following intravenous administration of alemtuzumab at a dose of 10 mg/kg on postpartum days 8–12. Serum levels of alemtuzumab were similar in lactating mice and offspring on postpartum day 13 and were associated with evidence of pharmacological activity (decrease in lymphocyte counts) in the offspring.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential prior to initiating CAMPATH therapy.
Contraception
Females
CAMPATH may cause embryo-fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)]. Advise female patients of reproductive potential to use effective contraception during treatment with CAMPATH and for at least 3 months after the last dose.
Infertility
Based on findings from animal studies, alemtuzumab may impair fertility in females and males of reproductive potential [see Nonclinical Toxicology (13.1)]. The reversibility of the effect on fertility is unknown.
8.5 Geriatric Use
Of 147 previously untreated B-CLL patients treated with CAMPATH, 35% were ≥ age 65 and 4% were ≥ age 75. Of 149 previously treated patients with B-CLL, 44% were ≥65 years of age and 10% were ≥75 years of age. Clinical studies of CAMPATH did not include sufficient number of subjects age 65 and over to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
10 OVERDOSAGE
Across all clinical experience, the reported maximum single dose received was 90 mg. Bone marrow aplasia, infections, or severe infusions reactions occurred in patients who received a dose higher than recommended.
One patient received an 80 mg dose by IV infusion and experienced acute bronchospasm, cough, and dyspnea, followed by anuria and death. Another patient received two 90 mg doses by IV infusion one day apart during the second week of treatment and experienced a rapid onset of bone marrow aplasia.
There is no known specific antidote for CAMPATH overdosage. Treatment consists of drug discontinuation and supportive therapy.
-
11 DESCRIPTION
CAMPATH (alemtuzumab) is a recombinant DNA-derived humanized monoclonal antibody (CAMPATH-1H) directed against the 21–28 kD cell surface glycoprotein, CD52. CAMPATH-1H is an IgG1 kappa antibody with human variable framework and constant regions, and complementarity-determining regions from a murine (rat) monoclonal antibody (CAMPATH-1G). The CAMPATH-1H antibody has an approximate molecular weight of 150 kD. CAMPATH is produced in mammalian cell (Chinese hamster ovary) suspension culture in a medium containing neomycin. Neomycin is not detectable in the final product.
CAMPATH is a sterile, clear, colorless, isotonic solution (pH 6.8–7.4) for injection. Each single-use vial of CAMPATH contains 30 mg alemtuzumab, 8.0 mg sodium chloride, 1.44 mg dibasic sodium phosphate, 0.2 mg potassium chloride, 0.2 mg monobasic potassium phosphate, 0.1 mg polysorbate 80, and 0.0187 mg disodium edetate dihydrate. No preservatives are added.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
CAMPATH binds to CD52, an antigen present on the surface of B and T lymphocytes, a majority of monocytes, macrophages, NK cells, and a subpopulation of granulocytes. A proportion of bone marrow cells, including some CD34+ cells, express variable levels of CD52. The proposed mechanism of action is antibody-dependent cellular-mediated lysis following cell surface binding of CAMPATH to the leukemic cells.
12.2 Pharmacodynamics
Cardiac Electrophysiology
The effect of multiple doses of alemtuzumab (12 mg/day for 5 days) on the QTc interval was evaluated in a single-arm study in 53 patients without malignancy. No large changes in the mean QTc interval (i.e. >20 ms) were detected in the study. A mean increase in heart rate of 22 to 26 beats/min was observed for at least 2 hours following the initial infusion of alemtuzumab. This increase in heart rate was not observed with subsequent doses.
12.3 Pharmacokinetics
CAMPATH pharmacokinetics were characterized in a study of 30 previously treated B-CLL patients in whom CAMPATH was administered at the recommended dose and schedule. CAMPATH pharmacokinetics displayed nonlinear elimination kinetics. After the last 30 mg dose, the mean volume of distribution at steady-state was 0.18 L/kg (range 0.1 to 0.4 L/kg). Systemic clearance decreased with repeated administration due to decreased receptor-mediated clearance (i.e. loss of CD52 receptors in the periphery). After 12 weeks of dosing, patients exhibited a seven-fold increase in mean AUC. Mean half-life was 11 hours (range 2 to 32 hours) after the first 30 mg dose and was 6 days (range 1 to 14 days) after the last 30 mg dose.
Comparisons of AUC in patients ≥65 years (n=6) versus patients <65 years (n=15) suggested that no dose adjustments are necessary for age. Comparisons of AUC in female patients (n=4) versus male patients (n=17) suggested that no dose adjustments are necessary for gender.
The pharmacokinetics of CAMPATH in pediatric patients has not been studied. The effects of renal or hepatic impairment on the pharmacokinetics of CAMPATH have not been studied.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies to assess the carcinogenic or genotoxic potential of CAMPATH have not been conducted.
In fertility studies, alemtuzumab (3 or 10 mg/kg IV) was administered to huCD52 transgenic male mice on 5 consecutive days prior to cohabitation with untreated wild-type females. No effect on fertility or reproductive performance was observed. However, adverse effects on sperm parameters (including abnormal morphology [detached/no head] and reduced total count and motility) were observed at both doses tested.
When alemtuzumab (3 or 10 mg/kg IV) was administered to huCD52 transgenic female mice for 5 consecutive days prior to cohabitation with untreated wild-type males, there was a decrease in the average number of corpora lutea and implantation sites and an increase in postimplantation loss, resulting in fewer viable embryos at the higher dose tested.
-
14 CLINICAL STUDIES
14.1 Previously Untreated B-CLL Patients
CAMPATH was evaluated in an open-label, randomized (1:1) active-controlled study in previously untreated patients with B-CLL, Rai Stage I–IV, with evidence of progressive disease requiring therapy. Patients received either CAMPATH 30 mg IV 3 times/week for a maximum of 12 weeks or chlorambucil 40 mg/m2 PO once every 28 days, for a maximum of 12 cycles.
Of the 297 patients randomized, the median age was 60 years, 72% were male, 99% were Caucasian, 96% had a WHO performance status 0–1, 23% had maximum lymph node diameter ≥5 cm, 34% were Rai Stage III/IV, and 8% were treated in the U.S.
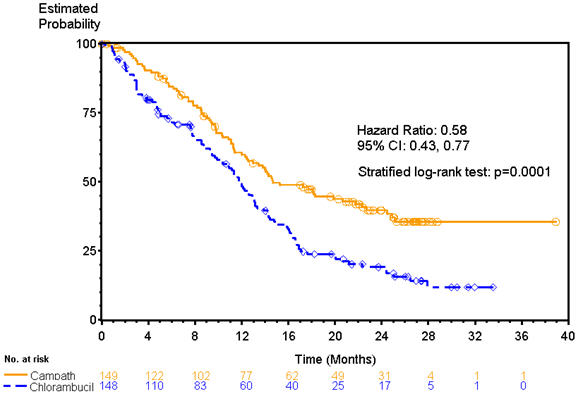
Patients randomized to receive CAMPATH experienced longer progression free survival (PFS) compared to those randomized to receive chlorambucil (median PFS 14.6 months vs. 11.7 months, respectively). The overall response rates were 83% and 55% (p <0.0001) and the complete response rates were 24% and 2% (p <0.0001) for CAMPATH and chlorambucil arms, respectively. The Kaplan-Meier curve for PFS is shown in Figure 1.
14.2 Previously Treated B-CLL Patients
CAMPATH was evaluated in three multicenter, open-label, single-arm studies of 149 patients with B-CLL previously treated with alkylating agents, fludarabine, or other chemotherapies. Patients were treated with the recommended dose of CAMPATH, 30 mg intravenously, three times per week for up to 12 weeks. Partial response rates of 21% to 31% and complete response rates of 0% to 2% were observed.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
CAMPATH (alemtuzumab) is supplied in single-use clear glass vials containing 30 mg of alemtuzumab in 1 mL of solution. Each carton contains three CAMPATH vials (NDC: 58468-0357-3) or one CAMPATH vial (NDC: 58468-0357-1).
-
17 PATIENT COUNSELING INFORMATION
Cytopenias: Advise patients to report any signs or symptoms such as bleeding, easy bruising, petechiae or purpura, pallor, weakness or fatigue [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
Infusion Reactions: Advise patients of the signs and symptoms of infusion reactions and of the need to take premedications as prescribed [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
Infections: Advise patients to immediately report symptoms of infection (e.g. pyrexia) and to take prophylactic anti-infectives for PCP (trimethoprim/sulfamethoxazole DS or equivalent) and for herpes virus (famciclovir or equivalent) as prescribed [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)].
Advise patients that irradiation of blood products is required [see Warnings and Precautions (5.3)].
Advise patients that they should not be immunized with live viral vaccines if they have recently been treated with CAMPATH. Advise females with infants exposed to CAMPATH in utero to inform the pediatrician of the exposure [see Warnings and Precautions (5.5)].
Embryo-fetal Toxicity: Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy. Advise female patients of reproductive potential to use effective contraception during treatment with CAMPATH and for 3 months after the final dose [see Use in Specific Populations (8.1, 8.3)].
Lactation: Advise females not to breastfeed during treatment with CAMPATH and for 3 months after the final dose [see Use in Specific Populations (8.2)].
Infertility: Advise females and males of reproductive potential that CAMPATH may impair fertility [see Use in Specific Populations (8.3) and Nonclinical Toxicology (13.1)].
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
Package Label – Principal Display Panel – 1-Pack Carton
NDC: 58468-0357-1
1 Single-Use 1 mL Vial
Campath
alemtuzumab
For Intravenous Use Only
30 mg/mL
- 30 mg/mL
- 1 vial
- Injection30 mg/mL
No US Standard of Potency
Must be further diluted prior to IV administration
Single-Use Vial, Discard Unused Portion
See package insert for full prescribing information
Sterile
Rx Only
Note: Concentration change to 30 mg/mL
See revised insert for new instructions for preparation and administration.
-
PRINCIPAL DISPLAY PANEL
Package Label – Principal Display Panel – 3-Pack Carton
NDC: 58468-0357-3
3 Single-Use 1 mL Vial
Campath
alemtuzumab
For Intravenous Use Only
30 mg/mL
- 30 mg/mL
- 3 vials
- Injection
30 mg/mL
No US Standard of Potency
Must be further diluted prior to IV administration
Single-Use Vial, Discard Unused Portion
See package insert for full prescribing information
Sterile
Rx Only
Note: Concentration change to 30 mg/mL
See revised insert for new instructions for preparation and administration.
-
INGREDIENTS AND APPEARANCE
CAMPATH
alemtuzumab injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 58468-0357 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALEMTUZUMAB (UNII: 3A189DH42V) (ALEMTUZUMAB - UNII:3A189DH42V) ALEMTUZUMAB 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 8 mg in 1 mL SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) 1.44 mg in 1 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) 0.2 mg in 1 mL POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) 0.2 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.1 mg in 1 mL EDETATE DISODIUM (UNII: 7FLD91C86K) 0.0187 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58468-0357-1 1 in 1 CARTON 11/30/2009 1 1 mL in 1.0 VIAL, SINGLE-USE; Type 0: Not a Combination Product 2 NDC: 58468-0357-3 3 in 1 CARTON 11/30/2009 2 1 mL in 1.0 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103948 11/30/2009 Labeler - Genzyme Corporation (025322157) Establishment Name Address ID/FEI Business Operations Boehringer Ingelheim Pharma GmbH & Co. KG (BI Pharma) 340700520 ANALYSIS(58468-0357) , API MANUFACTURE(58468-0357) , MANUFACTURE(58468-0357) Establishment Name Address ID/FEI Business Operations Sanofi-Aventis Deutschland GmbH 313218430 ANALYSIS(58468-0357) Establishment Name Address ID/FEI Business Operations Genzyme Limited 229522842 ANALYSIS(58468-0357) , LABEL(58468-0357) , PACK(58468-0357) Establishment Name Address ID/FEI Business Operations Genzyme Ireland Limited 985127419 ANALYSIS(58468-0357) , LABEL(58468-0357) , PACK(58468-0357)
Trademark Results [Campath]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CAMPATH 74717719 2251598 Live/Registered |
Murata Kikai Kabushiki Kaisha 1995-08-18 |
 CAMPATH 74169781 1767878 Live/Registered |
GENZYME CORPORATION 1991-05-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.