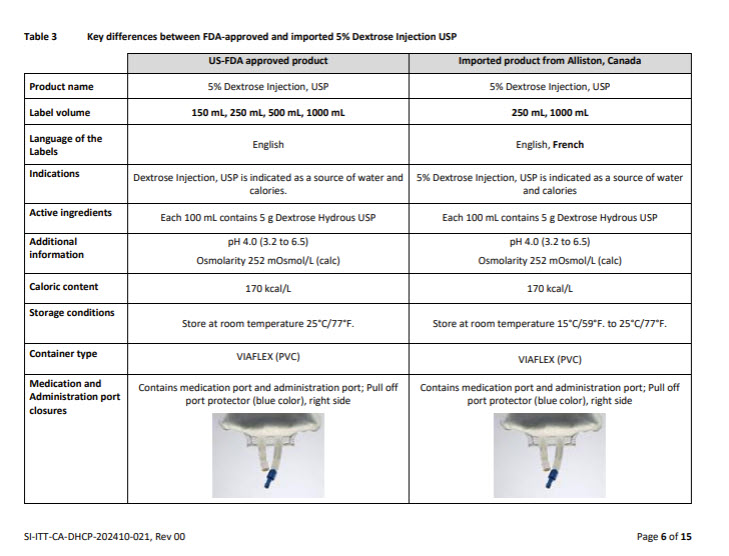
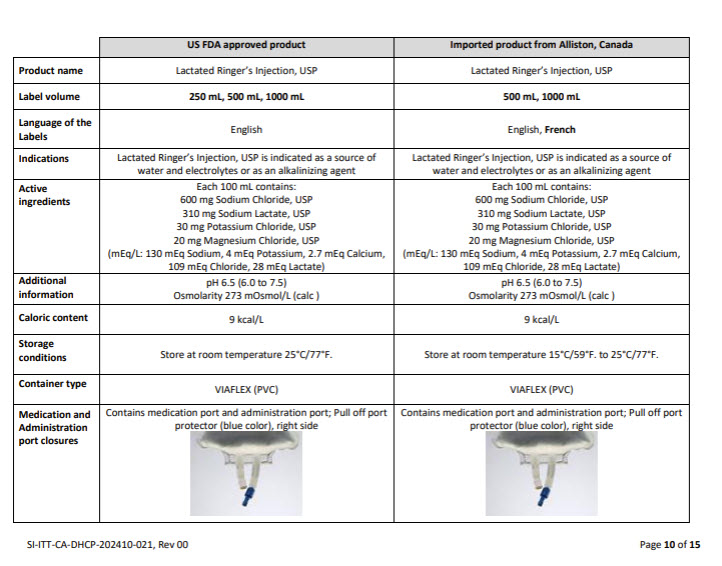
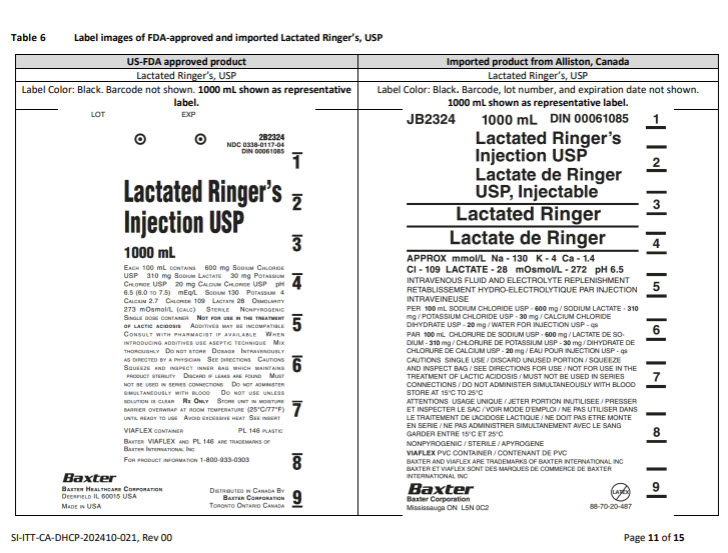
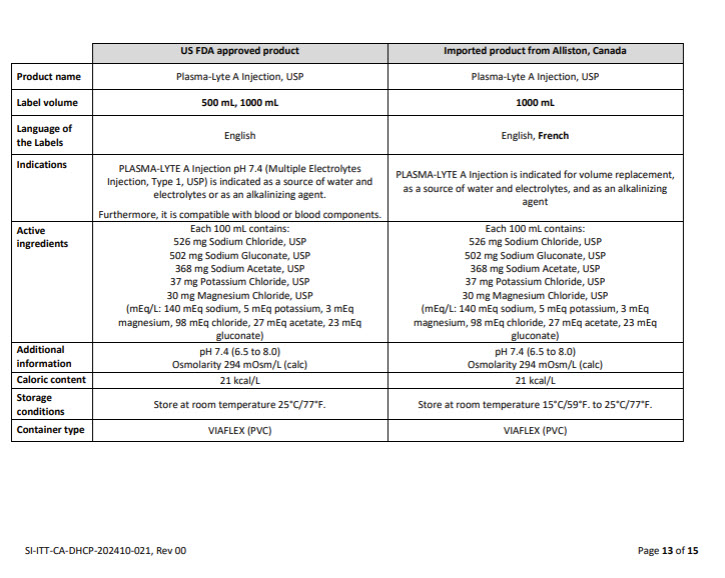
5% DEXTROSE INJECTION, USP
Dextrose by
Drug Labeling and Warnings
Dextrose by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Company, Baxter Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DEXTROSE- dextrose monohydrate injection, solution
Baxter Healthcare Company
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
5% DEXTROSE INJECTION, USP
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Container Label
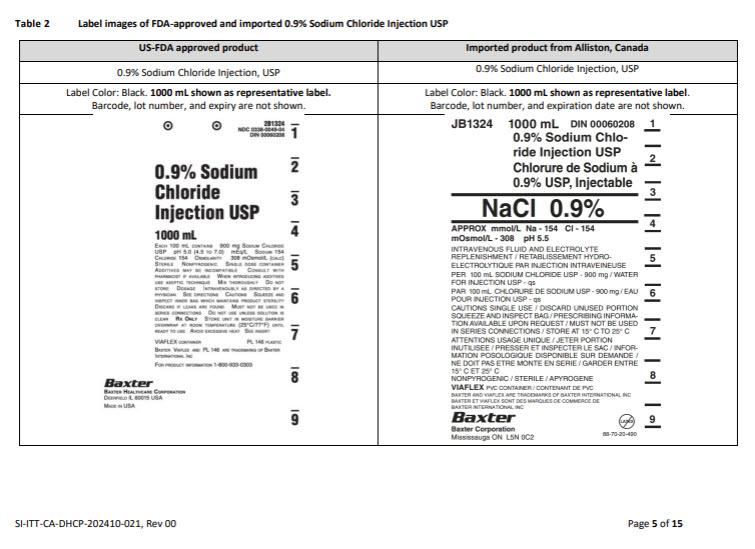
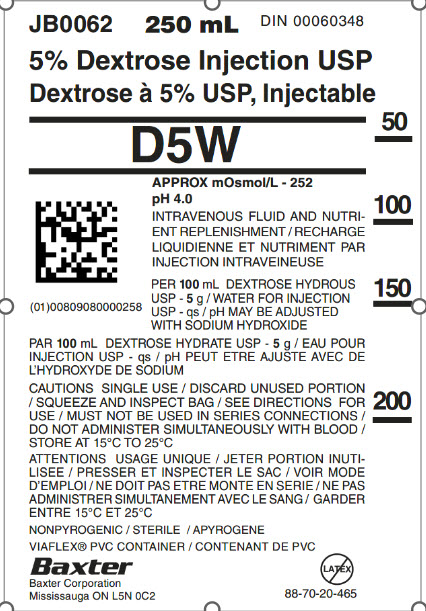
JB0062 250 mL DIN 00060348
5% Dextrose Injection USP
Dextrose à 5% USP, Injectable
D5W
2D Barcode
(01)00809080000258
APPROX mOsmol/L – 252
pH 4.0
INTRAVENOUS FLUID AND NUTRI-
ENT REPLENISHIMENT / RECHARGE
LIQUIDIENNE ET NUTRIMENT PAR
INJECTION INTRAVEINEUSE
PER 100 mL DEXTROSE HYDROUS
USP – 5 g / WATER FOR INJECTION
USP – qs / pH MAY BE ADJUSTED
WITH SODIUM HYDROXIDE
PAR 100 mL DEXTROSE HYDRATE USP – 5 g / EAU POUR
INJECTION USP – qs / pH PEUT ETRE AJUSTE AVEC DE
L’HYDROXYDE DE SODIUM
CAUTIONS SINGLE USE / DISCARD UNUSED PORTION
/ SQUEEZE AND INSPECT BAG / SEE DIRECTIONS FOR
USE / MUST NOT BE USED IN SERIES CONNECTIONS /
DO NOT ADMINISTER SIMULTANEOUSLY WITH BLOOD /
STORE AT 15°C TO 25°C
ATTENTIONS USAGE UNIQUE / JETER PORTION INTUI-
LISEE / PRESSER ET INSPECTOR LE SAC / VOIR MODE
D’EMPLOI / NE DOIT PAS ETRE MONTE EN SERIE / NE PAS
ADMINISTRER SIMULTANEMENT AVEC LA SANG / GARDER
ENTRE 15°C ET 25°C
NONPYROGENIC / STERILE / APYROGENE
VIAFLEX® PVC CONTAINER / CONTENANT DE PVC
Baxter Logo
Baxter Corporation
Mississauga ON L5N 0C2
No Latex Label
88-70-20-465
50
100
150
200
Container Label
JB0064 1000 mL DIN 00060348
5% Dextrose
Injection USP
Dextrose à 5%
USP, Injectable
D5W
APPROX mOsmol/L – 252 pH 4.0
INTRAVENOUS FLUID AND NUTRIENT REPLENISHIMENT /
RECHARGE LIQUIDIENNE ET NUTRIMENT PAR INJECTION
INTRAVEINEUSE
PER 100 mL DEXTROSE HYDROUS USP – 5 g / WATER FOR
INJECTION USP – qs / pH MAY BE ADJUSTED WITH SODIUM
HYDROXIDE
PAR 100 mL DEXTROSE HYDRATE USP – 5 g / EAU POUR
INJECTION USP – qs / pH PEUT ETRE AJUSTE AVEC DE
L’HYDROXYDE DE SODIUM
CAUTIONS SINGLE USE / DISCARD UNUSED PORTION
SQUEEZE AND INSPECT BAG / SEE DIRECTIONS FOR USE
MUST NOT BE USED IN SERIES CONNECTIONS / DO NOT
ADMINISTER SIMULTANEOUSLY WITH BLOOD / STORE AT
15°C TO 25°C
ATTENTIONS USAGE UNIQUE / JETER PORTION INTUILISEE
PRESSER ET INSPECTOR LE SAC / VOIR MODE D’EMPLOI NE
DOIT PAS ETRE MONTE EN SERIE / NE PAS ADMINISTRER
SIMULTANEMENT AVEC LA SANG / GARDER ENTRE 15°C
ET 25°C
NONPYROGENIC / STERILE / APYROGENE
VIAFLEX PVC CONTAINER / CONTENANT DE PVC
BAXTER AND VIAFLEX ARE TRADEMARKS OF BAXTER INTERNATIONAL INC
BAXTER ET VIAFLEX SONT DES MARQUES DE COMMERCE DE BAXTER
INTERNATIONAL INC
Baxter Logo
Baxter Corporation
Mississauga ON L5N 0C2
No Latex Label
07-25-77-316
1
_
2
_
3
_
4
_
5
_
6
_
7
_
8
_
9
| DEXTROSE
dextrose monohydrate injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| DEXTROSE
dextrose monohydrate injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Baxter Healthcare Company (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Corporation | 205087968 | ANALYSIS(0338-9830, 0338-9588) , LABEL(0338-9830, 0338-9588) , MANUFACTURE(0338-9830, 0338-9588) , STERILIZE(0338-9830, 0338-9588) , PACK(0338-9830, 0338-9588) | |