FOOTLOGIX MOISTURIZING DIABETIC FORMULA- dimethicone aerosol, foam
footlogix by
Drug Labeling and Warnings
footlogix by is a Otc medication manufactured, distributed, or labeled by KVG Group Inc . Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
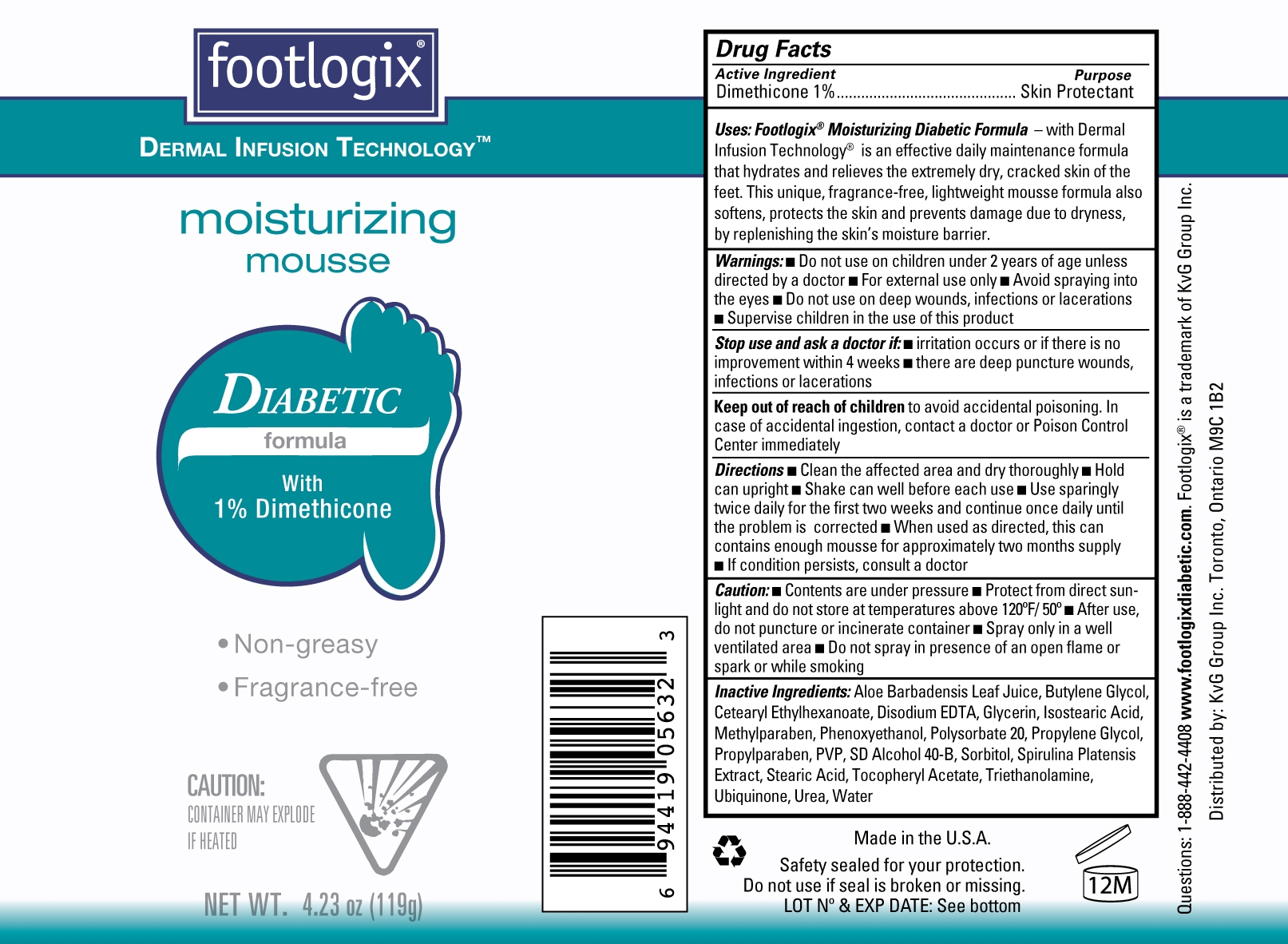
Uses: Footlogix Moisturizing Diabetic Formula- with Dermal Infusion Technology is an effective daily maintenance formula that hydrates and relieves the extremely dry, cracked skin of the feet. This unique fragrance-free, lightweight mousse formula also softens, protects the skni and prevents damage due to dryness by replenishing the skin's moisture barrier.
- WARNINGS
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions ■ Clean the affected area and dry thoroughly ■ Hold the can upright ■ Shake well before each use ■ Use sparingly twice daily for the first two weeks and continue once daily until the problem is corrected ■ When used as directed, this can contains enough mousse for approximately two months supply ■ If condition persists, consult a doctor
- SPL UNCLASSIFIED SECTION
-
INACTIVE INGREDIENT
Inactive Ingredients Aloe Barbadensis Leaf Juice, Butylene Glycol, Cetearyl Ethylhexanoate, Disodium EDTA, Glycerin, Isostearic Acid, Methylparaben, Phenoxyethanol, Polysorbate 20, Propylene Glycol, Propylparaben, PVP, SD Alcohol 40-B, Sorbitol, Spirulina Platensis Extract, Stearic Acid, Tocopheryl Acetate, Triethanolamine, Ubiquinone, Urea, Water.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FOOTLOGIX MOISTURIZING DIABETIC FORMULA
dimethicone aerosol, foamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 42479-214 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CETEARYL ETHYLHEXANOATE (UNII: 9M64UO4C25) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ISOSTEARIC ACID (UNII: X33R8U0062) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) POVIDONE (UNII: FZ989GH94E) SORBITOL (UNII: 506T60A25R) SPIRULINA PLATENSIS (UNII: 9L3TIH1UUE) STEARIC ACID (UNII: 4ELV7Z65AP) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TROLAMINE (UNII: 9O3K93S3TK) UREA (UNII: 8W8T17847W) WATER (UNII: 059QF0KO0R) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42479-214-04 119 g in 1 CAN Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 07/15/2012 Labeler - KVG Group Inc (206932605)
Trademark Results [footlogix]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 FOOTLOGIX 77020305 3544039 Live/Registered |
KVG GROUP INC. 2006-10-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.