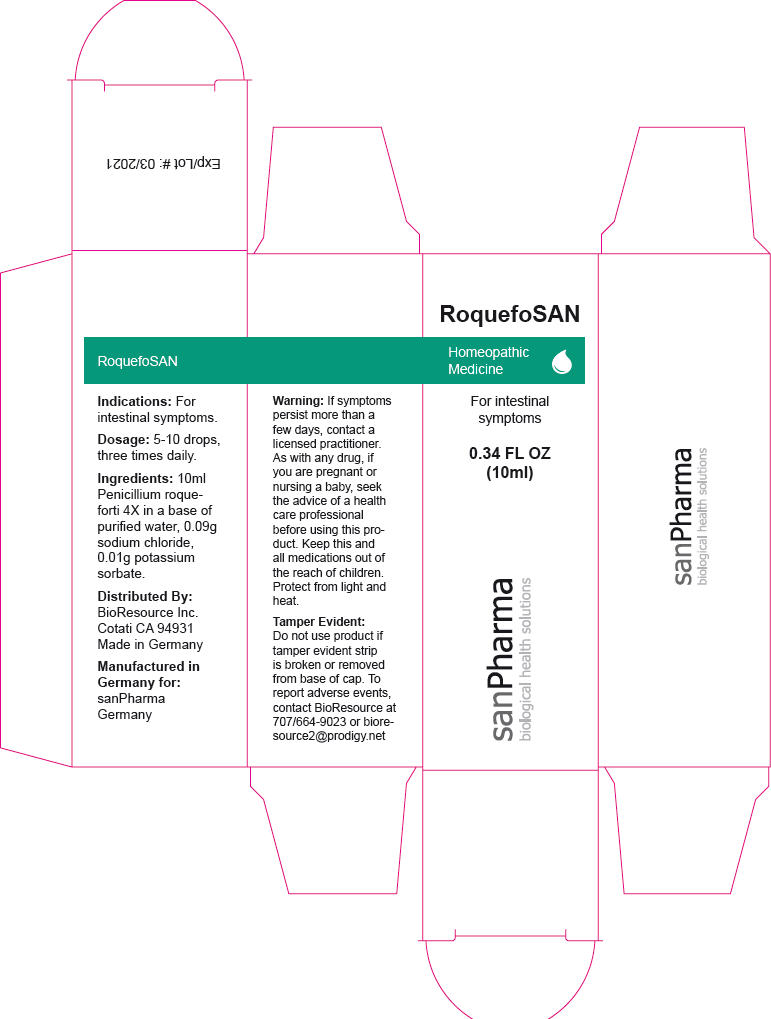
RoquefoSAN by sanPharma GmbH RoquefoSAN
RoquefoSAN by
Drug Labeling and Warnings
RoquefoSAN by is a Homeopathic medication manufactured, distributed, or labeled by sanPharma GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ROQUEFOSAN- penicillium roqueforti liquid
sanPharma GmbH
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
RoquefoSAN
Warning
If symptoms persist more than a few days, contact a licensed practitioner. As with any drug, if you are pregnant or nursing a baby, seek the advice of a health care professional before using this product.
| ROQUEFOSAN
penicillium roqueforti liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - sanPharma GmbH (341409153) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| sanPharma GmbH | 341409153 | manufacture(64232-035) , label(64232-035) | |
Revised: 12/2019
Document Id: 995887da-f5f9-5306-e053-2a95a90a8cfc
Set id: 500ef881-9a8e-4b80-9f8a-431fdb68e64e
Version: 4
Effective Time: 20191210
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.