NAPROXEN SODIUM TABLETS USP 220 MG (TABLET/CAPLET)
Naproxen Sodium by
Drug Labeling and Warnings
Naproxen Sodium by is a Other medication manufactured, distributed, or labeled by Granules India Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NAPROXEN SODIUM- naproxen sodium tablet, film coated
Granules India Limited
----------
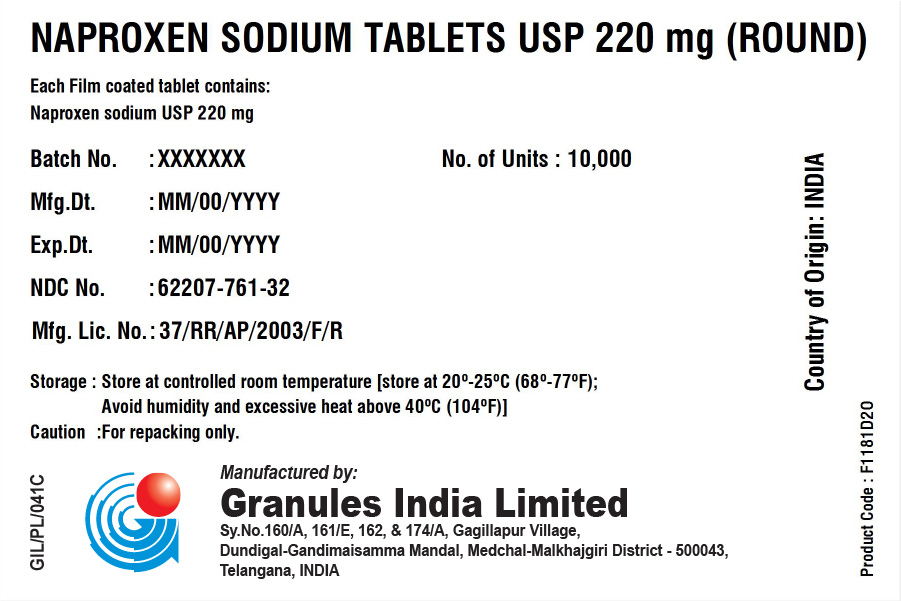
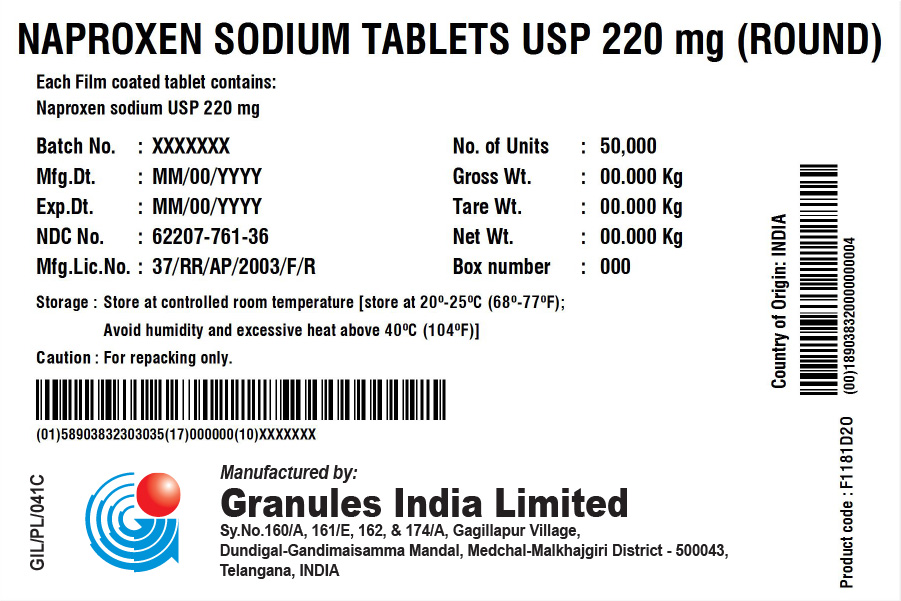
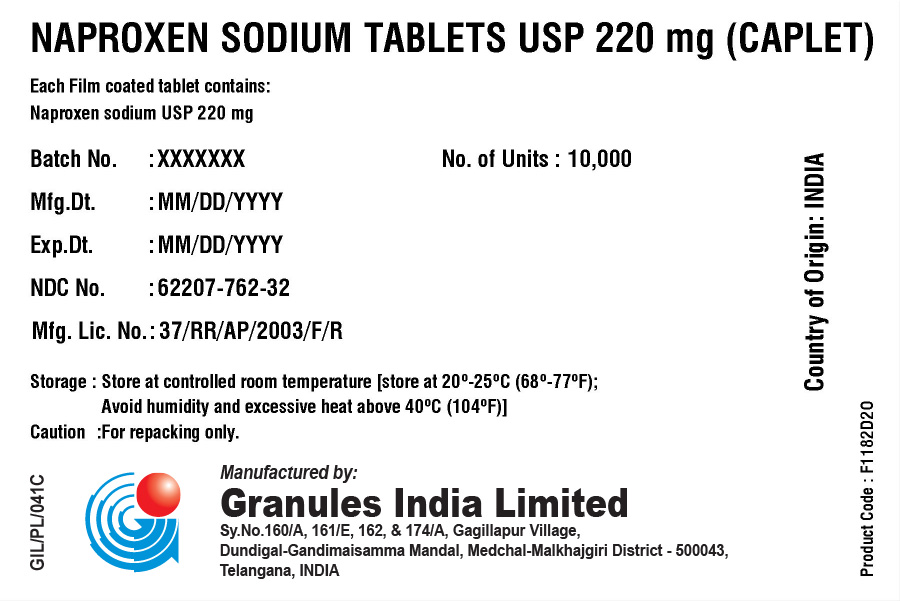
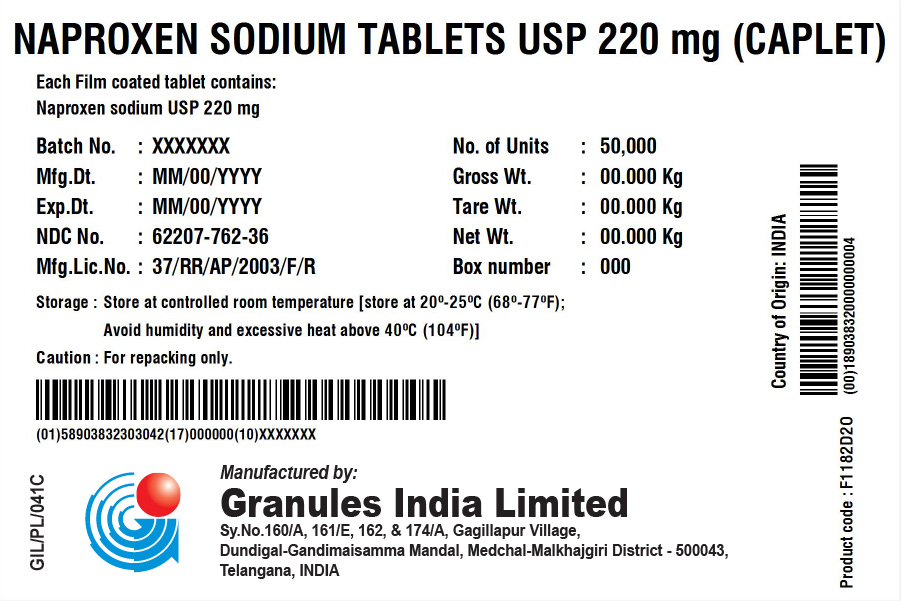
NAPROXEN SODIUM TABLETS USP 220 MG (TABLET/CAPLET)
Each film coated tablet/caplet contains
Naproxen Sodium USP 220 mg (naproxen 200 mg)
BOX
| Batch No.: | XXX | No. of Units | 50,000 (5 x 10,000) |
| Mfg. Dt.: | XXX | Gross Wt.: | XXX kg |
| Exp. Dt.: | XXX | Tare Wt.: | XXX kg |
| NDC No. | XXX | Net Wt.: | XXX kg |
| Mfg. Lic. No.: | 37/RR/AP/2003/F/R | Box No.: | XXX |
| Storage: | Store at controlled room temperature [store at 20° – 25°C (68° – 77°F); Avoid humidity and excessive heat above 40°C (104°F). | ||
| Caution: | For repacking only. | ||
POUCH
| Batch No.: | XXX | No. of Units | 10,000 |
| Mfg. Dt.: | XXX | ||
| Exp. Dt.: | XXX | ||
| NDC No. | XXX | ||
| Mfg. Lic. No.: | 37/RR/AP/2003/F/R | ||
| Storage: | Store at controlled room temperature [store at 20° – 25°C (68° – 77°F); Avoid humidity and excessive heat above 40°C (104°F). | ||
| Caution: | For repacking only. | ||
Country of Origin: INDIA
Product code: XXXXXX
Manufactured in India by:
Granules India Limited
Plot No. 160/A, 161/E, Gagillapur Village
Qutbullapur Mandal, Ranga Reddy Dt – 500 043, AP.
| NAPROXEN SODIUM
naproxen sodium tablet, film coated |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NAPROXEN SODIUM
naproxen sodium tablet, film coated |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Granules India Limited (915000087) |
| Registrant - Granules India Limited (915000087) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Granules India Limited | 918609236 | manufacture(62207-761, 62207-762) , analysis(62207-761, 62207-762) , pack(62207-761, 62207-762) | |
Revised: 1/2024
Document Id: 0e81a10f-9347-d142-e063-6394a90a28d0
Set id: 506161f7-3bce-4c4d-8c05-8f3b0d5f88df
Version: 8
Effective Time: 20240109
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.