IMPLANON- etonogestrel implant
IMPLANON by
Drug Labeling and Warnings
IMPLANON by is a Prescription medication manufactured, distributed, or labeled by Organon USA Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use IMPLANON safely and effectively. See full prescribing information for IMPLANON.
IMPLANON® (etonogestrel implant), for subdermal use
Initial U.S. Approval: 2001INDICATIONS AND USAGE
IMPLANON is a progestin indicated for use by women to prevent pregnancy. (1)
DOSAGE AND ADMINISTRATION
Insert one IMPLANON subdermally just under the skin at the inner side of the non-dominant upper arm. IMPLANON must be removed no later than by the end of the third year. (2)
DOSAGE FORMS AND STRENGTHS
IMPLANON consists of a single, rod-shaped implant, containing 68 mg etonogestrel, pre-loaded in the needle of a disposable applicator. (3)
CONTRAINDICATIONS
- Known or suspected pregnancy (4)
- Current or past history of thrombosis or thromboembolic disorders (4, 5.4)
- Liver tumors, benign or malignant, or active liver disease (4, 5.7)
- Undiagnosed abnormal genital bleeding (4, 5.2)
- Known or suspected breast cancer, personal history of breast cancer, or other progestin-sensitive cancer, now or in the past (4, 5.6)
- Allergic reaction to any of the components of IMPLANON (4, 6)
WARNINGS AND PRECAUTIONS
- Insertion and removal complications: Pain, paresthesias, bleeding, hematoma, scarring or infection may occur. (5.1)
- Menstrual bleeding pattern: Counsel women regarding changes in bleeding frequency, intensity, or duration. (5.2)
- Ectopic pregnancies: Be alert to the possibility of an ectopic pregnancy in women using IMPLANON who become pregnant or complain of lower abdominal pain. (5.3)
- Thrombotic and other vascular events: The IMPLANON implant should be removed in the event of a thrombosis. (5.4)
- Liver disease: Remove the IMPLANON implant if jaundice occurs. (5.7)
- Elevated blood pressure: The IMPLANON implant should be removed if blood pressure rises significantly and becomes uncontrolled. (5.9)
- Carbohydrate and lipid metabolic effects: Monitor prediabetic and diabetic women using IMPLANON. (5.11)
ADVERSE REACTIONS
Most common (≥10%) adverse reactions reported in clinical trials were change in menstrual bleeding pattern, headache, vaginitis, weight increase, acne, breast pain, abdominal pain, and pharyngitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Drugs or herbal products that induce certain enzymes, such as CYP3A4, may decrease the effectiveness of progestin hormonal contraceptives or increase breakthrough bleeding. (7.1)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Initiating Contraception with IMPLANON
2.2 Insertion of IMPLANON
2.3 Removal of IMPLANON
2.4 Replacing IMPLANON
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Complications of Insertion and Removal
5.2 Changes in Menstrual Bleeding Patterns
5.3 Ectopic Pregnancies
5.4 Thrombotic and Other Vascular Events
5.5 Ovarian Cysts
5.6 Carcinoma of the Breast and Reproductive Organs
5.7 Liver Disease
5.8 Weight Gain
5.9 Elevated Blood Pressure
5.10 Gallbladder Disease
5.11 Carbohydrate and Lipid Metabolic Effects
5.12 Depressed Mood
5.13 Return to Ovulation
5.14 Fluid Retention
5.15 Contact Lenses
5.16 In Situ Broken or Bent Implant
5.17 Monitoring
5.18 Drug-Laboratory Test Interactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Hormonal Contraceptives
7.2 Effects of Hormonal Contraceptives on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Overweight Women
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Pregnancy
14.2 Return to Ovulation
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
The efficacy of IMPLANON does not depend on daily, weekly or monthly administration.
All healthcare providers should receive instruction and training prior to performing insertion and/or removal of IMPLANON.
A single IMPLANON implant is inserted subdermally in the upper arm. To reduce the risk of neural or vascular injury, the implant should be inserted at the inner side of the non-dominant upper arm about 8-10 cm (3-4 inches) above the medial epicondyle of the humerus. The implant should be inserted subdermally just under the skin, avoiding the sulcus (groove) between the biceps and triceps muscles and the large blood vessels and nerves that lie there in the neurovascular bundle deeper in the subcutaneous tissues. An implant inserted more deeply than subdermally (deep insertion) may not be palpable and the localization and/or removal can be difficult or impossible [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)]. IMPLANON must be inserted by the expiration date stated on the packaging. IMPLANON is a long-acting (up to 3 years), reversible, hormonal contraceptive method. The implant must be removed by the end of the third year and may be replaced by a new implant at the time of removal, if continued contraceptive protection is desired.
2.1 Initiating Contraception with IMPLANON
IMPORTANT: Rule out pregnancy before inserting the implant.
Timing of insertion depends on the woman's recent contraceptive history, as follows:
-
No preceding hormonal contraceptive use in the past month
-
IMPLANON should be inserted between Day 1 (first day of menstrual bleeding) and Day 5 of the menstrual cycle, even if the woman is still bleeding.
-
If inserted as recommended, back-up contraception is not necessary. If deviating from the recommended timing of insertion, the woman should be advised to use a barrier method until 7 days after insertion. If intercourse has already occurred, pregnancy should be excluded.
-
IMPLANON should be inserted between Day 1 (first day of menstrual bleeding) and Day 5 of the menstrual cycle, even if the woman is still bleeding.
-
Switching contraceptive method to IMPLANON
-
Combination hormonal contraceptives:
IMPLANON should preferably be inserted on the day after the last active tablet of the previous combined oral contraceptive or on the day of the removal of the vaginal ring or transdermal patch. At the latest, IMPLANON should be inserted on the day following the usual tablet-free, ring-free, patch-free or placebo tablet interval of the previous combined hormonal contraceptive.
If inserted as recommended, back-up contraception is not necessary. If deviating from the recommended timing of insertion, the woman should be advised to use a barrier method until 7 days after insertion. If intercourse has already occurred, pregnancy should be excluded.
-
Progestin-only contraceptives:
There are several types of progestin-only methods. IMPLANON should be inserted as follows:
- Injectable Contraceptives: Insert IMPLANON on the day the next injection is due.
- Minipill: A woman may switch to IMPLANON on any day of the month. IMPLANON should be inserted within 24 hours after taking the last tablet.
- Contraceptive implant or intrauterine system (IUS): Insert IMPLANON on the same day as the previous contraceptive implant or IUS is removed.
If inserted as recommended, back-up contraception is not necessary. If deviating from the recommended timing of insertion, the woman should be advised to use a barrier method until 7 days after insertion. If intercourse has already occurred, pregnancy should be excluded. - Injectable Contraceptives: Insert IMPLANON on the day the next injection is due.
-
Combination hormonal contraceptives:
-
Following abortion or miscarriage
- First Trimester: IMPLANON should be inserted within 5 days following a first trimester abortion or miscarriage.
- Second Trimester: Insert IMPLANON between 21 to 28 days following second trimester abortion or miscarriage.
If inserted as recommended, back-up contraception is not necessary. If deviating from the recommended timing of insertion, the woman should be advised to use a barrier method until 7 days after insertion. If intercourse has already occurred, pregnancy should be excluded. - First Trimester: IMPLANON should be inserted within 5 days following a first trimester abortion or miscarriage.
-
Postpartum
-
Not Breastfeeding: IMPLANON should be inserted between 21 to 28 days postpartum. If inserted as recommended, back-up contraception is not necessary. If deviating from the recommended timing of insertion, the woman should be advised to use a barrier method until 7 days after insertion. If intercourse has already occurred, pregnancy should be excluded.
- Breastfeeding: IMPLANON should not be inserted until after the fourth postpartum week. The woman should be advised to use a barrier method until 7 days after insertion. If intercourse has already occurred, pregnancy should be excluded.
-
Not Breastfeeding: IMPLANON should be inserted between 21 to 28 days postpartum. If inserted as recommended, back-up contraception is not necessary. If deviating from the recommended timing of insertion, the woman should be advised to use a barrier method until 7 days after insertion. If intercourse has already occurred, pregnancy should be excluded.
2.2 Insertion of IMPLANON
The basis for successful use and subsequent removal of IMPLANON is a correct and carefully performed subdermal insertion of the single, rod-shaped implant in accordance with the instructions. Both the healthcare provider and the woman should be able to feel the implant under the skin after placement.
All healthcare providers performing insertions and/or removals of IMPLANON should receive instructions and training prior to inserting or removing the implant. Information concerning the insertion and removal of IMPLANON will be sent upon request free of charge [1-877-IMPLANON (1-877-467-5266)].
Preparation
Prior to inserting IMPLANON carefully read the instructions for insertion as well as the full prescribing information.
Before insertion of IMPLANON, the healthcare provider should confirm that:
- The woman is not pregnant nor has any other contraindication for the use of IMPLANON [see Contraindications (4)].
- The woman has had a medical history and physical examination, including a gynecologic examination, performed.
- The woman understands the benefits and risks of IMPLANON.
- The woman has received a copy of the Patient Labeling included in packaging.
- The woman has reviewed and completed a consent form to be maintained with the woman's chart.
- The woman does not have allergies to the antiseptic and anesthetic to be used during insertion.
Insert IMPLANON under aseptic conditions.
The following equipment is needed for the implant insertion:
- An examination table for the woman to lie on
- Sterile surgical drapes, sterile gloves, antiseptic solution, sterile marker (optional)
- Local anesthetic, needles, and syringe
- Sterile gauze, adhesive bandage, pressure bandage
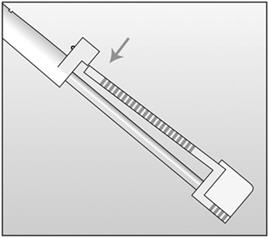
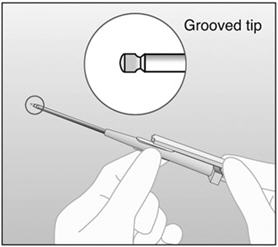
An applicator and its parts are shown below (Figures 1a and 1b).
Figure 1a (Not to scale) 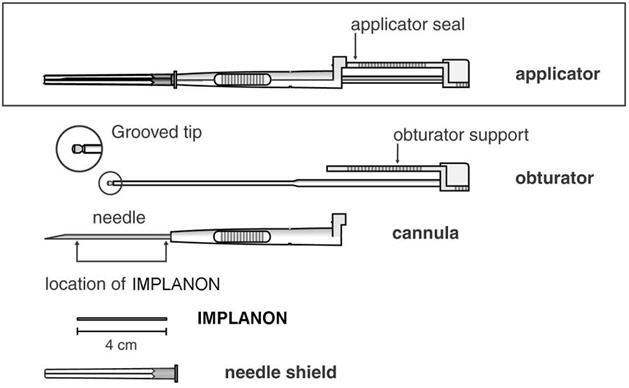
Figure 1b 
Grooved tip of obturator (enlarged) The procedure used for IMPLANON insertion is opposite from that of an injection. The obturator keeps IMPLANON in place while the cannula is retracted. The obturator must remain fixed in place while the cannula with needle is retracted from the arm. Do not push the obturator.
Insertion Procedure
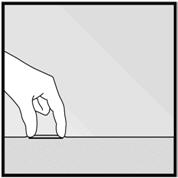
- Step 1. Have the woman lie on her back on the examination table with her non-dominant arm flexed at the elbow and externally rotated so that her wrist is parallel to her ear or her hand is positioned next to her head (Figure 2).
-
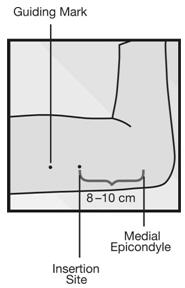
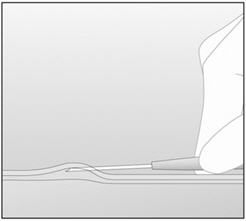
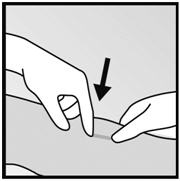
Step 2. Identify the insertion site, which is at the inner side of the non-dominant upper arm about 8-10 cm (3-4 inches) above the medial epicondyle of the humerus, avoiding the sulcus (groove) between the biceps and triceps muscles and the large blood vessels and nerves that lie there in the neurovascular bundle deeper in the subcutaneous tissue (Figure 3). The implant should be inserted subdermally just under the skin [see Warnings and Precautions (5.1)].
- Step 3. Make two marks with a sterile marker: first, mark the spot where the etonogestrel implant will be inserted, and second, mark a spot a few centimeters proximal to the first mark (Figure 3). This second mark will later serve as a direction guide during insertion.
- Step 4. Clean the insertion site with an antiseptic solution.
- Step 5. Anesthetize the insertion area (for example, with anesthetic spray or by injecting 2 mL of 1% lidocaine just under the skin along the planned insertion tunnel).
- Step 6. Remove the sterile pre-loaded disposable IMPLANON applicator carrying the implant from its blister. Keep the IMPLANON needle and rod sterile. The applicator should not be used if sterility is in question. If contamination occurs, use a new package of IMPLANON with a new sterile applicator.
- Step 7. Keep the shield on the needle and look for the IMPLANON rod, seen as a white cylinder inside the needle tip.
- Step 8. If you don't see the IMPLANON rod, tap the top of the needle shield against a firm surface to bring the implant into the needle tip.
- Step 9. Following visual confirmation, lower the IMPLANON rod back into the needle by tapping it back into the needle tip. Then remove the needle shield, while holding the applicator upright.
- Step 10. Note that IMPLANON can fall out of the needle. Therefore, after you remove the needle shield, keep the applicator in the upright position until the moment of insertion
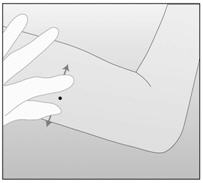
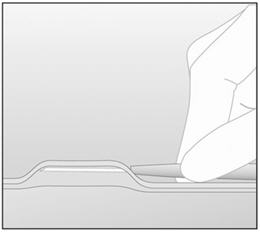
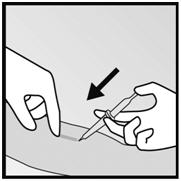
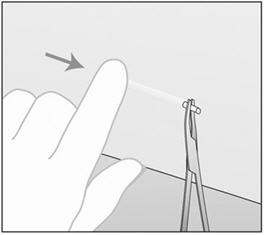
- Step 11. With your free hand, stretch the skin around the insertion site with thumb and index finger (Figure 4).
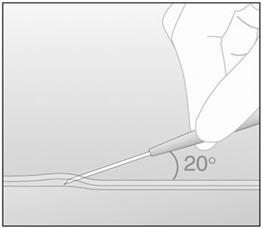
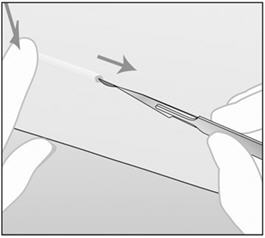
- Step 12. At a slight angle (not greater than 20°), insert only the tip of the needle with the beveled side up into the insertion site (Figure 5).
- Step 13. Lower the applicator to a horizontal position. Lift the skin up with the tip of the needle, but keep the needle in the subdermal connective tissue (Figure 6).
- Step 14. While "tenting" (lifting) the skin, gently insert the needle to its full length. Keep the needle parallel to the surface of the skin during insertion (Figure 7).
-
Step 15. If IMPLANON is placed deeply, the removal process can be difficult or impossible. If the needle is not inserted to its full length, the implant may protrude from the insertion site and fall out.
- Step 16. Break the seal of the applicator by pressing the obturator support (Figure 8).
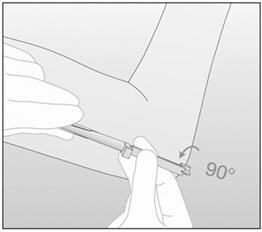
- Step 17. Turn the obturator 90° in either direction with respect to the needle (Figure 9).
- Step 18. While holding the obturator fixed in place on the arm, fully retract the cannula (Figure 10). Note: This procedure is opposite from an injection. Do not push the obturator. By holding the obturator fixed in place on the arm and fully retracting the cannula, the implant will be left in its correct subdermal position. Do not simultaneously retract the obturator and cannula from the patient's arm.
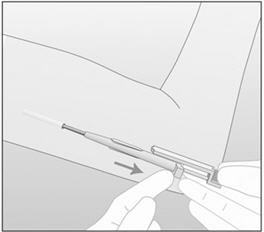
Figure 10 In this figure, the right hand is holding the obturator in place while the left hand is retracting the cannula. - Step 19. Confirm that the implant has been inserted by checking the tip of the needle for the absence of the implant. After insertion of the implant, the grooved tip of the obturator will be visible inside the needle (Figure 11).
-
Step 20. Always verify the presence of the implant in the woman's arm immediately after insertion by palpation. By palpating both ends of the implant, you should be able to confirm the presence of the 4-cm rod (Figure 12). See section below "If the rod is not palpable".
-
Step 21. Place a small adhesive bandage over the insertion site. Request that the woman palpate the implant.
-
Step 22. Apply a pressure bandage with sterile gauze to minimize bruising. The woman may remove the pressure bandage in 24 hours and the small bandage over the insertion site in 3 to 5 days.
-
Step 23. Complete the USER CARD and give it to the woman to keep. Also, complete the PATIENT CHART LABEL and affix it to the woman's medical record.
-
Step 24. The applicator is for single use only and should be disposed in accordance with the Center for Disease Control and Prevention guidelines for handling of hazardous waste.
If you cannot feel the implant or are in doubt of its presence, the implant may not have been inserted or it may have been inserted deeply:
- Check the tip of the needle for the absence of the implant. After insertion of the implant, the grooved tip of the obturator will be visible inside the needle.
- Use other methods to confirm the presence of the implant. Suitable methods to locate are: ultrasound (US) with a high-frequency linear array transducer (10 MHz or greater) or magnetic resonance imaging (MRI). Please note that the IMPLANON rod is not radiopaque and cannot be seen by X-ray or CT scan. If ultrasound and MRI fail, call 1-877-IMPLANON (1-877-467-5266) for information on the procedure for measuring etonogestrel blood levels.
Until the presence of the implant has been verified, the woman should be advised to use a non-hormonal contraceptive method, such as condoms.
Once the non-palpable implant has been located, removal is recommended [see Warnings and Precautions (5.1)].
2.3 Removal of IMPLANON
Preparation
Before initiating the removal procedure, the healthcare provider should carefully read the instructions for removal and consult the USER CARD and/or the PATIENT CHART LABEL for the location of the implant. The exact location of the implant in the arm should be verified by palpation. [See Dosage and Administration (2.3) and Localization and Removal of a Non-Palpable Implant.]
Procedure for Removal of an Implant that is Palpable
Before removal of the implant, the healthcare provider should confirm that:
- The woman does not have allergies to the antiseptic or anesthetic to be used.
Remove the implant under aseptic conditions.
The following equipment is needed for removal of the implant:
- An examination table for the woman to lie on
- Sterile surgical drapes, sterile gloves, antiseptic solution, sterile marker (optional)
- Local anesthetic, needles, and syringe
- Sterile scalpel, forceps (straight and curved mosquito)
- Skin closure, sterile gauze, adhesive bandage and pressure bandages
Removal Procedure
- Step 1. Clean the site where the incision will be made and apply an antiseptic. Locate the implant by palpation and mark the distal end (end closest to the elbow), for example, with a sterile marker (Figure 13).
- Step 2. Anesthetize the arm, for example, with 0.5 to 1 mL 1% lidocaine at the marked site where the incision will be made (Figure 14). Be sure to inject the local anesthetic under the implant to keep it close to the skin surface.
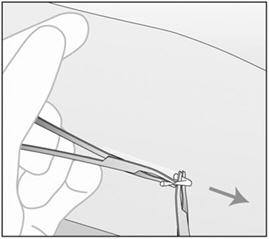
- Step 3. Push down the proximal end of the implant (Figure 15) to stabilize it; a bulge may appear indicating the distal end of the implant. Starting at the distal tip of the implant, make a longitudinal incision of 2 mm towards the elbow.
- Step 4. Gently push the implant towards the incision until the tip is visible. Grasp the implant with forceps (preferably curved mosquito forceps) and gently remove the implant (Figure 16).
- Step 5. If the implant is encapsulated, make an incision into the tissue sheath and then remove the implant with the forceps (Figures 17 and 18).
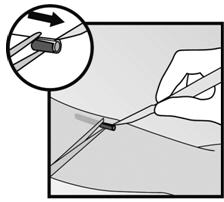
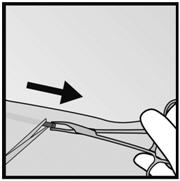
Figure 17 Figure 18 - Step 6. If the tip of the implant does not become visible in the incision, gently insert a forceps into the incision (Figure 19). Flip the forceps over into your other hand (Figure 20).
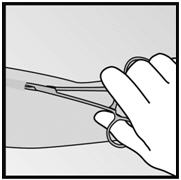
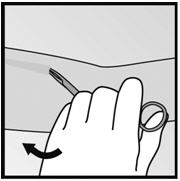
Figure 19 Figure 20 - Step 7. With a second pair of forceps carefully dissect the tissue around the implant and grasp the implant (Figure 21). The implant can then be removed.
-
Step 8. Confirm that the entire implant, which is 4 cm long, has been removed by measuring its length. There have been reports of broken implants while in the patient's arm. In some cases, difficult removal of the broken implant has been reported. If a partial implant (less than 4 cm) is removed, the remaining piece should be removed by following the instructions in section 2.3. [See Dosage and Administration (2.3).] If the woman would like to continue using IMPLANON, a new implant may be inserted immediately after the old implant is removed using the same incision [see Dosage and Administration (2.4)].
- Step 9. After removing the implant, close the incision with a steri-strip and apply an adhesive bandage.
- Step 10. Apply a pressure bandage with sterile gauze to minimize bruising. The woman may remove the pressure bandage in 24 hours and the small bandage in 3 to 5 days.
Localization and Removal of a Non-Palpable Implant
There have been reports of migration of the implant; usually this involves minor movement relative to the original position [see Warnings and Precautions (5.1)], but may lead to the implant not being palpable in the location in which it was placed. An implant that has been deeply inserted or has migrated may not be palpable and therefore imaging procedures, as described below, may be required for localization.
A non-palpable implant should always be located prior to attempting removal. Suitable methods for localization include ultrasound with a high-frequency linear array transducer (10 MHz or greater) or magnetic resonance imaging. Once the implant has been localized in the arm, the implant should be removed according to the instructions in Dosage and Administration (2.3), Procedure for Removal of an Implant that is Palpable, and the use of ultrasound guidance during the removal should be considered.
If the implant cannot be found in the arm after comprehensive localization attempts, consult a radiologist familiar with applying advanced imaging techniques to the chest, as events of migration to the pulmonary vasculature have been reported. If the implant is located in the chest, surgical or endovascular procedures may be needed for removal; healthcare providers familiar with the anatomy of the chest should be consulted.
If at any time these imaging methods fail to locate the implant, etonogestrel blood level determination can be used for verification of the presence of the implant. For details on etonogestrel blood level determination, call 1-877-IMPLANON (1-877-467-5266) for further instructions.
If the implant migrates within the arm, removal may require a minor surgical procedure with a larger incision or a surgical procedure in an operating room. Removal of deeply inserted implants should be conducted with caution in order to prevent injury to deeper neural or vascular structures in the arm and be performed by healthcare providers familiar with the anatomy of the arm.
Exploratory surgery without knowledge of the exact location of the implant is strongly discouraged.
2.4 Replacing IMPLANON
Immediate replacement can be done after removal of the previous implant and is similar to the insertion procedure described in section 2.2 Insertion of IMPLANON.
The new implant may be inserted in the same arm, and through the same incision from which the previous implant was removed. If the same incision is being used to insert a new implant, anesthetize the insertion site [for example, 2 mL lidocaine (1%)] applying it just under the skin along the 'insertion canal.'
Follow the subsequent steps in the insertion instructions [see Dosage and Administration (2.2)].
-
No preceding hormonal contraceptive use in the past month
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
IMPLANON should not be used in women who have
- Known or suspected pregnancy
- Current or past history of thrombosis or thromboembolic disorders
- Liver tumors, benign or malignant, or active liver disease
- Undiagnosed abnormal genital bleeding
- Known or suspected breast cancer, personal history of breast cancer, or other progestin-sensitive cancer, now or in the past
- Allergic reaction to any of the components of IMPLANON [see Adverse Reactions (6)]
-
5 WARNINGS AND PRECAUTIONS
The following information is based on experience with either IMPLANON, other progestin-only contraceptives, or experience with combination (estrogen plus progestin) oral contraceptives.
5.1 Complications of Insertion and Removal
IMPLANON should be inserted subdermally so that it will be palpable after insertion, and this should be confirmed by palpation immediately after insertion. Failure to insert IMPLANON properly may go unnoticed unless it is palpated immediately after insertion. Undetected failure to insert the implant may lead to an unintended pregnancy. Complications related to insertion and removal procedures, such as pain, paresthesias, bleeding, hematoma, scarring or infection, may occur. Occasionally in post-marketing use, implant insertions have failed because the implant fell out of the needle or remained in the needle during insertion.
If IMPLANON is inserted deeply (intramuscular or in the fascia), neural or vascular injury may occur. To reduce the risk of neural or vascular injury, IMPLANON should be inserted at the inner side of the non-dominant upper arm about 8-10 cm (3-4 inches) above the medial epicondyle of the humerus. IMPLANON should be inserted subdermally just under the skin, avoiding the sulcus (groove) between the biceps and triceps muscles and the large blood vessels and nerves that lie there in the neurovascular bundle deeper in the subcutaneous tissues. Deep insertions of IMPLANON have been associated with paraesthesia (due to neural injury), migration of the implant (due to intramuscular or fascial insertion), and intravascular insertion. If infection develops at the insertion site, start suitable treatment. If the infection persists, the implant should be removed. Incomplete insertions or infections may lead to expulsion.
Implant removal may be difficult or impossible if the implant is not inserted correctly, is inserted too deeply, not palpable, encased in fibrous tissue, or has migrated.
There have been reports of migration of the implant within the arm from the insertion site, which may be related to a deep insertion. There also have been postmarketing reports of implants located within the vessels of the arm and the pulmonary artery, which may be related to deep insertions or intravascular insertion. In cases where the implant has migrated to the pulmonary artery, endovascular procedures may be needed for removal.
If at any time the implant cannot be palpated, it should be localized and removal is recommended.
Exploratory surgery without knowledge of the exact location of the implant is strongly discouraged. Removal of deeply inserted implants should be conducted with caution in order to prevent injury to deeper neural or vascular structures in the arm and be performed by healthcare providers familiar with the anatomy of the arm. If the implant is located in the chest, healthcare providers familiar with the anatomy of the chest should be consulted. Failure to remove the implant may result in continued effects of etonogestrel, such as compromised fertility, ectopic pregnancy, or persistence or occurrence of a drug-related adverse event.
5.2 Changes in Menstrual Bleeding Patterns
After starting IMPLANON, women are likely to have a change from their normal menstrual bleeding pattern. These may include changes in bleeding frequency (absent, less, more frequent or continuous), intensity (reduced or increased) or duration. In clinical trials, bleeding patterns ranged from amenorrhea (1 in 5 women) to frequent and/or prolonged bleeding (1 in 5 women). The bleeding pattern experienced during the first three months of IMPLANON use is broadly predictive of the future bleeding pattern for many women. Women should be counseled regarding the bleeding pattern changes they may experience so that they know what to expect. Abnormal bleeding should be evaluated as needed to exclude pathologic conditions or pregnancy.
In clinical studies of IMPLANON, reports of changes in bleeding pattern were the most common reason for stopping treatment (11.1%). Irregular bleeding (10.8%) was the single most common reason women stopped treatment, while amenorrhea (0.3%) was cited less frequently. In these studies, women had an average of 17.7 days of bleeding or spotting every 90 days (based on 3,315 intervals of 90 days recorded by 780 patients). The percentages of patients having 0, 1-7, 8-21, or >21 days of spotting or bleeding over a 90-day interval while using the IMPLANON implant are shown in Table 1.
Table 1: Percentages of Patients with 0, 1 - 7, 8 - 21, or >21 Days of Spotting or Bleeding Over a 90-Day Interval While Using IMPLANON Total Days of Spotting or Bleeding Percentage of Patients Treatment Days 91-180
(N = 745)Treatment Days
271-360
(N = 657)Treatment Days
631-720
(N = 547)0 Days 19% 24% 17% 1-7 Days 15% 13% 12% 8-21 Days 30% 30% 37% >21 Days 35% 33% 35% Bleeding patterns observed with use of IMPLANON for up to 2 years, and the proportion of 90-day intervals with these bleeding patterns, are summarized in Table 2.
Table 2: Bleeding Patterns Using IMPLANON during the First 2 Years of Use* BLEEDING PATTERNS DEFINITIONS %† - * Based on 3,315 recording periods of 90 day's duration in 780 women, excluding the first 90 days after implant insertion
- † % = Percentage of 90-day intervals with this pattern
Infrequent Less than three bleeding and/or spotting episodes in 90 days (excluding amenorrhea) 33.6 Amenorrhea No bleeding and/or spotting in 90 days 22.2 Prolonged Any bleeding and/or spotting episode lasting more than 14 days in 90 days 17.7 Frequent More than 5 bleeding and/or spotting episodes in 90 days 6.7 In case of undiagnosed, persistent, or recurrent abnormal vaginal bleeding, appropriate measures should be conducted to rule out malignancy.
5.3 Ectopic Pregnancies
As with all progestin-only contraceptive products, be alert to the possibility of an ectopic pregnancy among women using IMPLANON who become pregnant or complain of lower abdominal pain. Although ectopic pregnancies are uncommon among women using IMPLANON, a pregnancy that occurs in a woman using IMPLANON may be more likely to be ectopic than a pregnancy occurring in a woman using no contraception.
5.4 Thrombotic and Other Vascular Events
The use of combination hormonal contraceptives (progestin plus estrogen) increases the risk of vascular events, including arterial events (strokes and myocardial infarctions) or deep venous thrombotic events (venous thromboembolism, deep venous thrombosis, retinal vein thrombosis, and pulmonary embolism). IMPLANON is a progestin-only contraceptive. It is unknown whether this increased risk is applicable to etonogestrel alone. It is recommended, however, that women with risk factors known to increase the risk of venous and arterial thromboembolism be carefully assessed.
There have been postmarketing reports of serious arterial thrombotic and venous thromboembolic events, including cases of pulmonary emboli (some fatal), deep vein thrombosis, myocardial infarction, and strokes, in women using IMPLANON. IMPLANON should be removed in the event of a thrombosis.
Due to the risk of thromboembolism associated with pregnancy and immediately following delivery, IMPLANON should not be used prior to 21 days postpartum. Women with a history of thromboembolic disorders should be made aware of the possibility of a recurrence.
Evaluate for retinal vein thrombosis immediately if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions.
Consider removal of the IMPLANON implant in case of long-term immobilization due to surgery or illness.
5.5 Ovarian Cysts
If follicular development occurs, atresia of the follicle is sometimes delayed, and the follicle may continue to grow beyond the size it would attain in a normal cycle. Generally, these enlarged follicles disappear spontaneously. On rare occasion, surgery may be required.
5.6 Carcinoma of the Breast and Reproductive Organs
Women who currently have or have had breast cancer should not use hormonal contraception because breast cancer may be hormonally sensitive [see Contraindications (4)]. Some studies suggest that the use of combination hormonal contraceptives might increase the incidence of breast cancer; however, other studies have not confirmed such findings.
Some studies suggest that the use of combination hormonal contraceptives is associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there is controversy about the extent to which such findings are due to differences in sexual behavior and other factors.
Women with a family history of breast cancer or who develop breast nodules should be carefully monitored.
5.7 Liver Disease
Disturbances of liver function may necessitate the discontinuation of hormonal contraceptive use until markers of liver function return to normal. Remove IMPLANON if jaundice develops.
Hepatic adenomas are associated with combination hormonal contraceptives use. An estimate of the attributable risk is 3.3 cases per 100,000 for combination hormonal contraceptives users. It is not known whether a similar risk exists with progestin-only methods like IMPLANON.
The progestin in IMPLANON may be poorly metabolized in women with liver impairment. Use of IMPLANON in women with active liver disease or liver cancer is contraindicated [see Contraindications (4)].
5.8 Weight Gain
In clinical studies, mean weight gain in US IMPLANON users was 2.8 pounds after 1 year and 3.7 pounds after 2 years. How much of the weight gain was related to the implant is unknown. In studies, 2.3% of the users reported weight gain as the reason for having the implant removed.
5.9 Elevated Blood Pressure
Women with a history of hypertension-related diseases or renal disease should be discouraged from using hormonal contraception. For women with well-controlled hypertension, use of IMPLANON can be considered. Women with hypertension using IMPLANON should be closely monitored. If sustained hypertension develops during the use of IMPLANON, or if a significant increase in blood pressure does not respond adequately to antihypertensive therapy, IMPLANON should be removed.
5.10 Gallbladder Disease
Studies suggest a small increased relative risk of developing gallbladder disease among combination hormonal contraceptive users. It is not known whether a similar risk exists with progestin-only methods like IMPLANON.
5.11 Carbohydrate and Lipid Metabolic Effects
Use of IMPLANON may induce mild insulin resistance and small changes in glucose concentrations of unknown clinical significance. Carefully monitor prediabetic and diabetic women using IMPLANON.
Women who are being treated for hyperlipidemia should be followed closely if they elect to use IMPLANON. Some progestins may elevate LDL levels and may render the control of hyperlipidemia more difficult.
5.12 Depressed Mood
Women with a history of depressed mood should be carefully observed. Consideration should be given to removing IMPLANON in patients who become significantly depressed.
5.13 Return to Ovulation
In clinical trials with IMPLANON, the etonogestrel levels in blood decreased below sensitivity of the assay by one week after removal of the implant. In addition, pregnancies were observed to occur as early as 7 to 14 days after removal. Therefore, a woman should re-start contraception immediately after removal of the implant if continued contraceptive protection is desired.
5.14 Fluid Retention
Hormonal contraceptives may cause some degree of fluid retention. They should be prescribed with caution, and only with careful monitoring, in patients with conditions which might be aggravated by fluid retention. It is unknown if IMPLANON causes fluid retention.
5.15 Contact Lenses
Contact lens wearers who develop visual changes or changes in lens tolerance should be assessed by an ophthalmologist.
5.16 In Situ Broken or Bent Implant
There have been reports of broken or bent implants while in the patient's arm. Based on in vitro data, when the implant is broken or bent, the release rate of etonogestrel may be slightly increased.
When an implant is removed, it is important to remove it in its entirety [see Dosage and Administration (2.3)].
-
6 ADVERSE REACTIONS
The following adverse reactions reported with the use of hormonal contraception are discussed elsewhere in the labeling:
- Changes in Menstrual Bleeding Patterns [see Warnings and Precautions (5.2)]
- Ectopic Pregnancies [see Warnings and Precautions (5.3)]
- Thrombotic and Other Vascular Events [see Warnings and Precautions (5.4)]
- Liver Disease [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials including 942 women who were evaluated for safety, change in menstrual bleeding patterns (irregular menses) was the most common adverse reaction causing discontinuation of use of IMPLANON (11.1% of women).
Adverse reactions that resulted in a rate of discontinuation of ≥1% are shown in Table 3.
Table 3: Adverse Reactions Leading to Discontinuation of Treatment in 1% or More of Subjects in Clinical Trials of IMPLANON Adverse Reactions All Studies
N = 942- * Includes "frequent", "heavy", "prolonged", "spotting", and other patterns of bleeding irregularity.
- † Among US subjects (N=330), 6.1% experienced emotional lability that led to discontinuation.
- ‡ Among US subjects (N=330), 2.4% experienced depression that led to discontinuation.
Bleeding Irregularities* 11.1% Emotional Lability† 2.3% Weight Increase 2.3% Headache 1.6% Acne 1.3% Depression‡ 1.0% Other adverse reactions that were reported by at least 5% of subjects in clinical trials of IMPLANON are listed in Table 4.
Table 4: Common Adverse Reactions Reported by ≥5% of Subjects in Clinical Trials with IMPLANON Adverse Reaction All Studies
N=942Headache 24.9% Vaginitis 14.5% Weight increase 13.7% Acne 13.5% Breast pain 12.8% Abdominal pain 10.9% Pharyngitis 10.5% Leukorrhea 9.6% Influenza-like symptoms 7.6% Dizziness 7.2% Dysmenorrhea 7.2% Back pain 6.8% Emotional lability 6.5% Nausea 6.4% Pain 5.6% Nervousness 5.6% Depression 5.5% Hypersensitivity 5.4% Insertion site pain 5.2% Implant site complications were reported by 3.6% of subjects during any of the assessments in clinical trials. Pain was the most frequent implant site complication, reported during and/or after insertion, occurring in 2.9% of subjects. Additionally, hematoma, redness, and swelling were reported by 0.1%, 0.3%, and 0.3% of patients, respectively [see Warnings and Precautions (5.1)].
6.2 Postmarketing Experience
The following additional adverse reactions have been identified during post-approval use of IMPLANON. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal disorders: constipation, diarrhea, flatulence, vomiting.
General disorders and administration site conditions: edema, fatigue, implant site reaction, pyrexia.
Immune system disorders: anaphylactic reactions
Infections and infestations: rhinitis, urinary tract infection.
Investigations: clinically relevant rise in blood pressure, weight decreased.
Metabolism and nutrition disorders: increased appetite.
Musculoskeletal and connective tissue disorders: arthralgia, musculoskeletal pain, myalgia.
Nervous system disorders: convulsions, migraine, somnolence.
Pregnancy, puerperium and perinatal conditions: ectopic pregnancy.
Psychiatric disorders: anxiety, insomnia, libido decreased.
Renal and urinary disorders: dysuria.
Reproductive system and breast disorders: breast discharge, breast enlargement, ovarian cyst, pruritus genital, vulvovaginal discomfort.
Skin and subcutaneous tissue disorders: angioedema, aggravation of angioedema and/or aggravation of hereditary angioedema, alopecia, chloasma, hypertrichosis, pruritus, rash, seborrhea, urticaria.
Vascular disorders: hot flush.
Complications related to insertion or removal of the implant reported include: bruising, slight local irritation, pain or itching, fibrosis at the implant site, paresthesia or paresthesia-like events, scarring and abscess. Expulsion or migration of the implant has been reported, including to the chest wall. In some cases implants have been found within the vasculature including the pulmonary artery. Some cases of implants found within the pulmonary artery reported chest pain and/or dyspnea; others have been reported as asymptomatic [see Warnings and Precautions (5.1)]. Surgical intervention might be necessary when removing the implant.
-
7 DRUG INTERACTIONS
Consult the labeling of concurrently-used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations.
7.1 Effects of Other Drugs on Hormonal Contraceptives
Substances decreasing the plasma concentrations of hormonal contraceptives (HCs) and potentially diminishing the efficacy of HCs:
Drugs or herbal products that induce certain enzymes, including cytochrome P450 3A4 (CYP3A4), may decrease the plasma concentrations of HCs and potentially diminish the effectiveness of HCs or increase breakthrough bleeding.
Some drugs or herbal products that may decrease the effectiveness of HCs include efavirenz, phenytoin, barbiturates, carbamazepine, bosentan, felbamate, griseofulvin, oxcarbazepine, rifampicin, topiramate, rifabutin, rufinamide, aprepitant, and products containing St. John's wort. Interactions between HCs and other drugs may lead to breakthrough bleeding and/or contraceptive failure. Counsel women to use an alternative non-hormonal method of contraception or a back-up method when enzyme inducers are used with HCs, and to continue back-up non-hormonal contraception for 28 days after discontinuing the enzyme inducer to ensure contraceptive reliability.
Substances increasing the plasma concentrations of HCs:
Co-administration of certain HCs and strong or moderate CYP3A4 inhibitors such as itraconazole, voriconazole, fluconazole, grapefruit juice, or ketoconazole may increase the serum concentrations of progestins, including etonogestrel.
Human Immunodeficiency Virus (HIV)/Hepatitis C Virus (HCV) protease inhibitors and non-nucleoside reverse transcriptase inhibitors:
Significant changes (increase or decrease) in the plasma concentrations of progestin have been noted in cases of co-administration with HIV protease inhibitors (decrease [e.g., nelfinavir, ritonavir, darunavir/ritonavir, (fos)amprenavir/ritonavir, lopinavir/ritonavir, and tipranavir/ritonavir] or increase [e.g., indinavir and atazanavir/ritonavir])/HCV protease inhibitors (decrease [e.g., boceprevir and telaprevir]) or with non-nucleoside reverse transcriptase inhibitors (decrease [e.g., nevirapine, efavirenz] or increase [e.g., etravirene]). These changes may be clinically relevant in some cases.
Consult the prescribing information of anti-viral and anti-retroviral concomitant medications to identify potential interactions.
7.2 Effects of Hormonal Contraceptives on Other Drugs
Hormonal contraceptives may affect the metabolism of other drugs. Consequently, plasma concentrations may either increase (for example, cyclosporine) or decrease (for example, lamotrigine). Consult the labeling of all concurrently-used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
IMPLANON is contraindicated during pregnancy because there is no need for pregnancy prevention in a woman who is already pregnant [see Contraindications (4)]. Epidemiologic studies and meta-analyses have not shown an increased risk of genital or non-genital birth defects (including cardiac anomalies and limb-reduction defects) following maternal exposure to low dose CHCs prior to conception or during early pregnancy. No adverse development outcomes were observed in pregnant rats and rabbits with the administration of etonogestrel during organogenesis at doses of 315 or 781 times the anticipated human dose (60 µg/day) (see Data).
IMPLANON should be removed if maintaining a pregnancy.
8.2 Lactation
Risk Summary
Small amounts of contraceptive steroids and/or metabolites, including etonogestrel are present in human milk. No significant adverse effects have been observed in the production or quality of breast milk, or on the physical and psychomotor development of breastfed infants (see Data).
Hormonal contraceptives, including etonogestrel, can reduce milk production in breastfeeding mothers. This is less likely to occur once breastfeeding is well-established; however, it can occur at any time in some women. When possible, advise the nursing mother about both hormonal and non-hormonal contraceptive options, as steroids may not be the initial choice for these patients. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for IMPLANON and any potential adverse effects on the breastfed child from IMPLANON or from the underlying maternal condition.
Data
The amount of etonogestrel contained within breast milk was measured in 38 lactating women who began using IMPLANON during the fourth to eighth week postpartum. The study evaluated Implanon versus another contraceptive, was not randomized and data were considered observational and exploratory; therefore, comparisons could not be made. Based on the findings of this study, during the first months after insertion of IMPLANON, when maternal blood levels of etonogestrel are highest, about 100 ng of etonogestrel may be ingested by the child per day based on an average daily milk ingestion of 658 mL. Based on daily milk ingestion of 150 mL/kg, the mean daily infant etonogestrel dose one month after insertion of IMPLANON is about 2.2% of the weight-adjusted maternal daily dose, or about 0.2% of the estimated absolute maternal daily dose. Adverse reactions were not observed in breastfed infants exposed to etonogestrel through breast milk. No adverse effects on the production or quality of breast milk were detected.
8.4 Pediatric Use
Safety and efficacy of IMPLANON have been established in women of reproductive age. Safety and efficacy of IMPLANON are expected to be the same for postpubertal adolescents. However, no clinical studies have been conducted in women less than 18 years of age. Use of this product before menarche is not indicated.
8.5 Geriatric Use
This product has not been studied in women over 65 years of age and is not indicated in this population.
8.6 Hepatic Impairment
No studies were conducted to evaluate the effect of hepatic disease on the disposition of IMPLANON. The use of IMPLANON in women with active liver disease is contraindicated [see Contraindications (4)].
8.7 Overweight Women
The effectiveness of IMPLANON in women who weighed more than 130% of their ideal body weight has not been defined because such women were not studied in clinical trials. Serum concentrations of etonogestrel are inversely related to body weight and decrease with time after implant insertion. It is therefore possible that IMPLANON may be less effective in overweight women, especially in the presence of other factors that decrease serum etonogestrel concentrations such as concomitant use of hepatic enzyme inducers.
- 10 OVERDOSAGE
-
11 DESCRIPTION
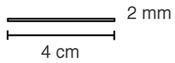
IMPLANON (etonogestrel implant) is a progestin-only, soft, flexible implant preloaded in a sterile, disposable applicator for subdermal use. The implant is off-white, non-biodegradable and 4 cm in length with a diameter of 2 mm (see Figure 22). Each implant consists of an ethylene vinylacetate (EVA) copolymer core, containing 68 mg of the synthetic progestin etonogestrel, surrounded by an EVA copolymer skin. Once inserted subdermally, the release rate is 60 to 70 mcg/day in Week 5 to 6 and decreases to approximately 35 to 45 mcg/day at the end of the first year, to approximately 30 to 40 mcg/day at the end of the second year, and then to approximately 25 to 30 mcg/day at the end of the third year. IMPLANON is a progestin-only contraceptive and does not contain estrogen. IMPLANON does not contain latex and is not radio-opaque.
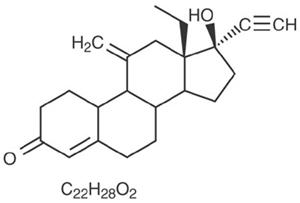
Etonogestrel [13-Ethyl-17-hydroxy-11-methylene-18,19-dinor-17α-pregn-4-en-20-yn-3-one], structurally derived from 19-nortestosterone, is the synthetic biologically active metabolite of the synthetic progestin desogestrel. It has a molecular weight of 324.46 and the following structural formula (Figure 23).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The contraceptive effect of IMPLANON is achieved by suppression of ovulation, increased viscosity of the cervical mucus, and alterations in the endometrium.
12.3 Pharmacokinetics
Absorption
After subdermal insertion of the etonogestrel implant, etonogestrel is released into the circulation and is approximately 100% bioavailable.
The mean peak serum concentrations in 3 pharmacokinetic studies ranged between 781 and 894 pg/mL and were reached within the first few weeks after insertion. The mean serum etonogestrel concentration decreases gradually over time declining to 192 to 261 pg/mL at 12 months (n=41), 154 to 194 pg/mL at 24 months (n=35), and 156 to 177 pg/mL at 36 months (n=17).
The pharmacokinetic profile of IMPLANON from 1 of 3 pharmacokinetic studies is shown in Figure 24.
Figure 24 Mean Serum Concentration-time Profile of Etonogestrel During 2 Years of IMPLANON Use and After Removal in 20 Healthy Women 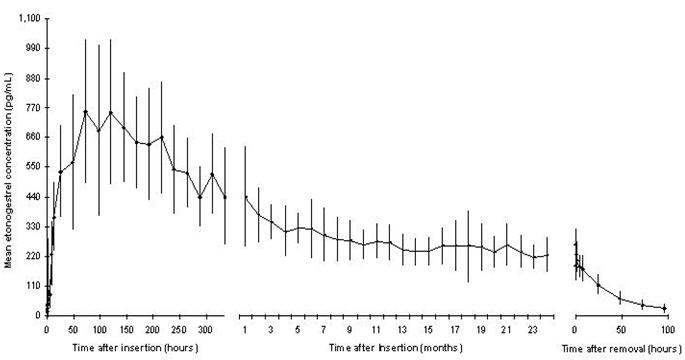
Figure 24 Distribution
The apparent volume of distribution averages about 201 L. Etonogestrel is approximately 32% bound to sex hormone binding globulin (SHBG) and 66% bound to albumin in blood.
Metabolism
In vitro data shows that etonogestrel is metabolized in liver microsomes by the cytochrome P450 3A4 isoenzyme. The biological activity of etonogestrel metabolites is unknown.
Excretion
The elimination half-life of etonogestrel is approximately 25 hours. Excretion of etonogestrel and its metabolites, either as free steroid or as conjugates, is mainly in urine and to a lesser extent in feces. After removal of the implant, etonogestrel concentrations decreased below sensitivity of the assay by 1 week.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 24-month carcinogenicity study in rats with subdermal implants releasing 10 and 20 mcg etonogestrel per day (equal to approximately 1.8-3.6 times the systemic steady state exposure in women using IMPLANON), no drug-related carcinogenic potential was observed. Etonogestrel was not genotoxic in the in vitro Ames/Salmonella reverse mutation assay, the chromosomal aberration assay in Chinese hamster ovary cells or in the in vivo mouse micronucleus test. Fertility returned after withdrawal from treatment.
-
14 CLINICAL STUDIES
14.1 Pregnancy
In clinical trials of up to 3 years duration that involved 923 subjects, 18 - 40 years of age at entry, and 1,756 women-years of IMPLANON use, the total exposures expressed as 28-day cycle equivalents by study year were:
- Year 1: 10,866 cycles
- Year 2: 8,581 cycles
- Year 3: 3,442 cycles
The clinical trials excluded women who:
- Weighed more than 130% of their ideal body weight
- Were chronically taking medications that induce liver enzymes
In the subgroup of women 18 to 35 years of age at entry, 6 pregnancies during 20,648 cycles of use were reported. Two pregnancies occurred in each of Years 1, 2 and 3. Each conception was likely to have occurred shortly before or within 2 weeks after IMPLANON removal. With these 6 pregnancies, the cumulative Pearl Index was 0.38 pregnancies per 100 women-years of use.
14.2 Return to Ovulation
In clinical trials with IMPLANON, the etonogestrel levels in blood decreased below sensitivity of the assay by one week after removal of the implant. In addition, pregnancies were observed to occur as early as 7 to 14 days after removal. Therefore, a woman should re-start contraception immediately after removal of the implant if continued contraceptive protection is desired.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
One IMPLANON package consists of a single implant containing 68 mg etonogestrel that is 4 cm in length and 2 mm in diameter, which is pre-loaded in the needle of a disposable applicator. The sterile applicator containing the implant is packed in a blister pack.
NDC: 0052-0272-01
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Counsel women about the insertion and removal procedure of the IMPLANON implant. Provide the woman with a copy of the Patient Labeling and ensure that she understands the information in the Patient Labeling before insertion and removal. A USER CARD and consent form are included in the packaging. Have the woman complete a consent form and retain it in your records. The USER CARD should be filled out and given to the patient after insertion of the IMPLANON implant so that she will have a record of the location of the implant in the upper arm and when it should be removed.
- Counsel women to contact their healthcare provider immediately if, at any time, they are unable to palpate the implant.
- Counsel women that IMPLANON does not protect against HIV infection (AIDS) or other sexually transmitted diseases.
- Counsel women that the use of IMPLANON may be associated with changes in their normal menstrual bleeding patterns so that they know what to expect.
-
SPL UNCLASSIFIED SECTION
Manufactured for: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USAManufactured by: N.V. Organon, Oss, The Netherlands, a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ 08889, USA
For patent information: www.merck.com/product/patent/home.html
Copyright © 2006-2017 Merck Sharp & Dohme B.V., a subsidiary of Merck & Co., Inc.
All rights reserved.uspi-mk8415-ipt-1705r014
-
PATIENT PACKAGE INSERT
FDA-Approved Patient Labeling
IMPLANON® (etonogestrel implant)
Subdermal Use
IMPLANON® does not protect against HIV infection (the virus that causes AIDS) or other sexually transmitted diseases. Read this Patient Information leaflet carefully before you decide if IMPLANON is right for you. This information does not take the place of talking with your healthcare provider. If you have any questions about IMPLANON, ask your healthcare provider.
What is IMPLANON?
IMPLANON is a hormone-releasing birth control implant for use by women to prevent pregnancy for up to 3 years. The implant is a flexible plastic rod about the size of a matchstick that contains a progestin hormone called etonogestrel. Your healthcare provider will insert the implant just under the skin of the inner side of your upper arm. You can use a single IMPLANON implant for up to 3 years. IMPLANON does not contain estrogen.

What if I need birth control for more than 3 years?
The IMPLANON implant must be removed after 3 years. Your healthcare provider can insert a new implant under your skin after taking out the old one if you choose to continue using IMPLANON for birth control.
What if I change my mind about birth control and want to stop using IMPLANON before 3 years?
Your healthcare provider can remove the implant at any time. You may become pregnant as early as the first week after removal of the implant. If you do not want to get pregnant after your healthcare provider removes the IMPLANON implant, you should start another birth control method right away.
How does IMPLANON work?
IMPLANON prevents pregnancy in several ways. The most important way is by stopping the release of an egg from your ovary. IMPLANON also thickens the mucus in your cervix and this change may keep sperm from reaching the egg. IMPLANON also changes the lining of your uterus.
How well does IMPLANON work?
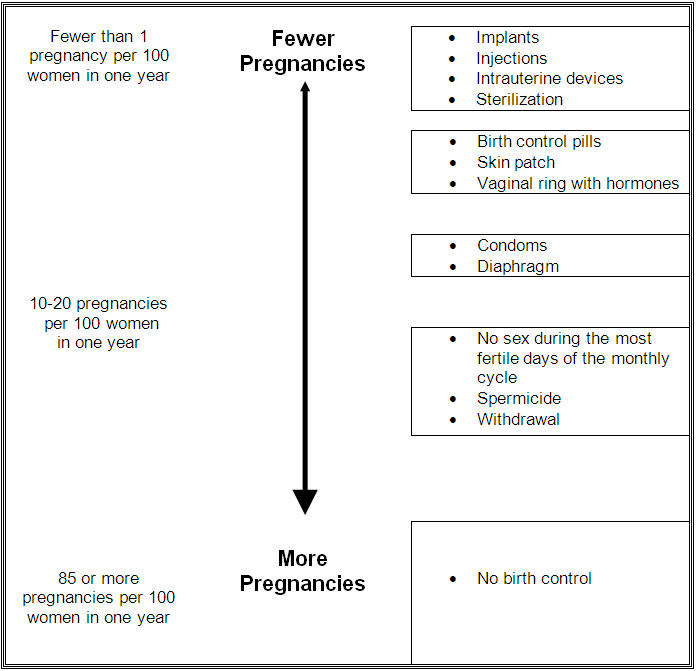
When the IMPLANON implant is placed correctly, your chance of getting pregnant is very low (less than 1 pregnancy per 100 women who use IMPLANON for 1 year). It is not known if IMPLANON is as effective in very overweight women because studies did not include many overweight women.
The following chart shows the chance of getting pregnant for women who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for women who do not use birth control and are trying to get pregnant.
Who should not use IMPLANON?
Do not use IMPLANON if you
- Are pregnant or think you may be pregnant
- Have, or have had serious blood clots, such as blood clots in your legs (deep venous thrombosis), lungs (pulmonary embolism), eyes (total or partial blindness), heart (heart attack), or brain (stroke)
- Have liver disease or a liver tumor
- Have unexplained vaginal bleeding
- Have breast cancer or any other cancer that is sensitive to progestin (a female hormone), now or in the past
- Are allergic to anything in IMPLANON
Tell your healthcare provider if you have or have had any of the conditions listed above. Your healthcare provider can suggest a different method of birth control.
In addition, talk to your healthcare provider about using IMPLANON if you:
- Have diabetes
- Have high cholesterol or triglycerides
- Have headaches
- Have gallbladder or kidney problems
- Have a history of depressed mood
- Have high blood pressure
- Have an allergy to numbing medicines (anesthetics) or medicines used to clean your skin (antiseptics). These medicines will be used when the implant is placed into or removed from your arm.
Interaction with Other Medicines
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. Certain medicines may make IMPLANON less effective, including:
- aprepitant
- barbiturates
- bosentan
- carbamazepine
- felbamate
- griseofulvin
- oxcarbazepine
- phenytoin
- rifampin
- St. John's wort
- topiramate
- HIV medicines
- Hepatitis C Virus medicines
Ask your healthcare provider if you are not sure if your medicine is one listed above.
If you are taking medicines or herbal products that might make IMPLANON less effective, you and your doctor may decide to leave IMPLANON in place; in that case, an additional non-hormonal contraceptive should be used. Because the effect of another medicine on IMPLANON may last up to 28 days after stopping the medicine, it is necessary to use the additional non-hormonal contraceptive for that long.
When you are using IMPLANON, tell all of your healthcare providers that you have IMPLANON in place in your arm.
How is the IMPLANON implant placed and removed?
Your healthcare provider will place and remove the IMPLANON implant in a minor surgical procedure in his or her office. The implant is placed just under the skin on the inner side of your upper arm.
The timing of insertion is important. Your healthcare provider may:
- Perform a pregnancy test before inserting IMPLANON
- Schedule the insertion at a specific time of your menstrual cycle (for example, within the first days of your regular menstrual bleeding)
Your healthcare provider will cover the site where IMPLANON was placed with 2 bandages. Leave the top bandage on for 24 hours. Keep the smaller bandage clean, dry, and in place for 3 to 5 days.
Immediately after the IMPLANON implant has been placed, you and your healthcare provider should check that the implant is in your arm by feeling for it.
If you cannot feel the implant immediately after insertion, the implant may not have been inserted, or it may have been inserted deeply. A deep insertion may cause problems with locating and removing the implant. Once the healthcare professional has located the implant, removal may be recommended.
If at any time you cannot feel the IMPLANON implant, contact your healthcare provider immediately and use a non-hormonal birth control method (such as condoms) until your healthcare provider confirms that the implant is in place. You may need special tests to check that the implant is in place or to help find the implant when it is time to take it out.
You will be asked to review and sign a consent form prior to inserting the IMPLANON implant. You will also get a USER CARD to keep at home with your health records. Your healthcare provider will fill out the USER CARD with the date the implant was inserted and the date the implant is to be removed. Keep track of the date the implant is to be removed. Schedule an appointment with your healthcare provider to remove the implant on or before the removal date.
Be sure to have checkups as advised by your healthcare provider.
What are the most common side effects I can expect while using IMPLANON?
-
Changes in Menstrual Bleeding Patterns (menstrual periods)
The most common side effect of IMPLANON is a change in your normal menstrual bleeding pattern. In studies, about one out of ten women stopped using the implant because of an unfavorable change in their bleeding pattern. You may experience longer or shorter bleeding during your periods or have no bleeding at all. The time between periods may vary, and in between periods you may also have spotting.
Talk with your healthcare provider right away if:
- You think you may be pregnant
- Your menstrual bleeding is heavy and prolonged
Besides changes in menstrual bleeding patterns, other frequent side effects that caused women to stop using the implant include:
- Mood swings
- Weight gain
- Headache
- Acne
- Depressed mood
Other common side effects include:
- Headache
- Vaginitis (inflammation of the vagina)
- Weight gain
- Acne
- Breast pain
- Viral infections such as sore throats or flu-like symptoms
- Stomach pain
- Painful periods
- Mood swings, nervousness, or depressed mood
- Back pain
- Nausea
- Dizziness
- Pain
- Pain at the site of insertion
Implants have been reported to be found in a blood vessel including a blood vessel in the lung.
This is not a complete list of possible side effects. For more information, ask your healthcare provider for advice about any side effects that concern you. You may report side effects to the FDA at 1-800-FDA-1088.
What are the possible risks of using IMPLANON?
-
Problems with Insertion and Removal
The implant may not be placed in your arm at all due to a failed insertion or if the implant has fallen out of the needle. If this happens, you may become pregnant. Immediately after insertion, and with help from your healthcare provider, you should be able to feel the implant under your skin. If you can't feel the implant, tell your healthcare provider.
Location and removal of the implant may be difficult or impossible because the implant is not where it should be. Special procedures, including surgery in the hospital, may be needed to remove the implant. If the implant is not removed, then the effects of IMPLANON will continue for a longer period of time.
Implants have been found in the pulmonary artery (a blood vessel in the lung). If the implant cannot be found in the arm, your healthcare professional may use imaging methods on the chest. If the implant is located in the chest, surgery may be needed.
Other problems related to insertion and removal are:- Pain, irritation, swelling, or bruising at the insertion site
- Scarring, including a thick scar called a keloid around the insertion site
- Infection
- Scar tissue may form around the implant making it difficult to remove
- The implant may come out by itself. You may become pregnant if the implant comes out by itself. Use a back-up birth control method and call your healthcare provider right away if the implant comes out.
- The need for surgery in the hospital to remove the implant
- Injury to nerves or blood vessels in your arm
- The implant breaks making removal difficult
-
Ectopic Pregnancy
If you become pregnant while using IMPLANON, you have a slightly higher chance that the pregnancy will be ectopic (occurring outside the womb) than do women who do not use birth control. Unusual vaginal bleeding or lower stomach (abdominal) pain may be a sign of ectopic pregnancy. Ectopic pregnancy is a medical emergency that often requires surgery. Ectopic pregnancies can cause serious internal bleeding, infertility, and even death. Call your healthcare provider right away if you think you are pregnant or have unexplained lower stomach (abdominal) pain. -
Ovarian Cysts
Cysts may develop on the ovaries and usually go away without treatment but sometimes surgery is needed to remove them. -
Breast Cancer
It is not known whether IMPLANON use changes a woman's risk for breast cancer. If you have breast cancer now, or have had it in the past, do not use IMPLANON because some breast cancers are sensitive to hormones. -
Serious Blood Clots
IMPLANON may increase your chance of serious blood clots, especially if you have other risk factors such as smoking. It is possible to die from a problem caused by a blood clot, such as a heart attack or a stroke.
Some examples of serious blood clots are blood clots in the:- Legs (deep vein thrombosis)
- Lung (pulmonary embolism)
- Brain (stroke)
- Heart (heart attack)
- Eyes (total or partial blindness)
The risk of serious blood clots is increased in women who smoke. If you smoke and want to use IMPLANON, you should quit. Your healthcare provider may be able to help.
Tell your healthcare provider at least 4 weeks before if you are going to have surgery or will need to be on bed rest. You have an increased chance of getting blood clots during surgery or bed rest. -
Other Risks
A few women who use birth control that contains hormones may get:- High blood pressure
- Gallbladder problems
- Rare cancerous or noncancerous liver tumors
-
Broken or Bent Implant
If you feel that the implant may have broken or bent while in your arm, contact your healthcare provider.
When should I call my healthcare provider?
Call your healthcare provider right away if you have:
- Pain in your lower leg that does not go away
- Severe chest pain or heaviness in the chest
- Sudden shortness of breath, sharp chest pain, or coughing blood
- Symptoms of a severe allergic reaction, such as swollen face, tongue or throat; trouble breathing or swallowing
- Sudden severe headache unlike your usual headaches
- Weakness or numbness in your arm, leg, or trouble speaking
- Sudden partial or complete blindness
- Yellowing of your skin or whites of your eyes, especially with fever, tiredness, loss of appetite, dark colored urine, or light colored bowel movements
- Severe pain, swelling, or tenderness in the lower stomach (abdomen)
- Lump in your breast
- Problems sleeping, lack of energy, tiredness, or you feel very sad
- Heavy menstrual bleeding
What if I become pregnant while using IMPLANON?
You should see your healthcare provider right away if you think that you may be pregnant. It is important to remove the implant and make sure that the pregnancy is not ectopic (occurring outside the womb). Based on experience with other hormonal contraceptives, IMPLANON is not likely to cause birth defects.
Can I use IMPLANON when I am breastfeeding?
If you are breastfeeding your child, you may use IMPLANON if 4 weeks have passed since you had your baby. A small amount of the hormone contained in IMPLANON passes into your breast milk. The health of breast-fed children whose mothers were using the implant has been studied up to 3 years of age in a small number of children. No effects on the growth and development of the children were seen. If you are breastfeeding and want to use IMPLANON, talk with your healthcare provider for more information.
Additional Information
This Patient Information leaflet contains important information about IMPLANON. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider for information about IMPLANON that is written for healthcare professionals. You may also call 1-877-IMPLANON (1-877-467-5266) or visit www.IMPLANON-USA.com
-
SPL UNCLASSIFIED SECTION
Manufactured for: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USAManufactured by: N.V. Organon, Oss, The Netherlands, a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ 08889, USA
For patent information: www.merck.com/product/patent/home.html
Copyright © 2012-2017 Merck Sharp & Dohme B.V., a subsidiary of Merck & Co., Inc.
All rights reserved.Revised: 05/2017
usppi-mk8415-ipt-1705r013
-
SPL UNCLASSIFIED SECTION
IMPLANON®
(etonogestrel implant)
68 mg For Subdermal Use OnlyPATIENT CONSENT FORM
I understand the Patient Labeling for IMPLANON®. I have discussed IMPLANON with my healthcare provider who answered all my questions. I understand that there are benefits as well as risks from using IMPLANON. I understand that there are other birth control methods and that each has its own benefits and risks.
I also understand that this Patient Consent Form is important. I understand that I need to sign this form to show that I am making an informed and careful decision to use IMPLANON, and that I have read and understand the following points.
- IMPLANON helps to keep me from getting pregnant.
- No contraceptive method is 100% effective, including IMPLANON.
- IMPLANON is made of a hormone mixed in a plastic rod.
- It is important to have IMPLANON inserted at the right time of my menstrual cycle.
- After IMPLANON is inserted, I should check that it is in place by gently pressing my fingertips over the skin in my arm where IMPLANON was inserted. I should be able to feel the small rod.
- IMPLANON must be removed at the end of 3 years. IMPLANON can be removed sooner if I want.
- If I have trouble finding a healthcare provider to remove IMPLANON, I can call (877) 467-5266 for help.
- IMPLANON is placed under the skin of my arm during a procedure done in my healthcare provider's office. There is a slight risk of getting a scar or an infection from this procedure.
- Removal is usually a small office procedure. However, removal may be difficult. Rarely, IMPLANON cannot be found when it is time to remove it. Special procedures, including surgery in the hospital, may be needed. Difficult removals may cause pain and scarring and may result in damage to nerves and blood vessels. If IMPLANON cannot be found, its effects may continue.
- Most women have changes in their menstrual bleeding while using IMPLANON. I also will likely have changes in my menstrual bleeding while using IMPLANON. My bleeding may be irregular, lighter or heavier, or my bleeding may completely stop. If I think I am pregnant, I should see my healthcare provider as soon as possible.
- I understand the warning signs for problems with IMPLANON. I should seek medical attention if any warning signs appear.
- I should tell all my healthcare providers that I am using IMPLANON.
- I need to have a medical checkup regularly and at any time I am having problems.
- IMPLANON does not protect me from HIV infection (AIDS) or any other sexually transmitted disease.
After learning about IMPLANON, I choose to use IMPLANON.
_______________________________ (Name of Healthcare Provider)
_______________________________ _______________ (Patient Signature) (Date) WITNESSED BY:
The patient above has signed this consent in my presence after I counseled her and answered her questions.
_______________________________ _______________ (Healthcare Provider Signature) (Date) I have provided an accurate translation of this information to the patient whose signature appears above. She has stated that she understands the information and has had an opportunity to have her questions answered.
_______________________________ ______________ (Signature of Translator) (Date) -
SPL UNCLASSIFIED SECTION
Manufactured for: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USAManufactured by: N.V. Organon, Oss, The Netherlands, a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ 08889, USA
For patent information: www.merck.com/product/patent/home.html
Copyright © 2006, 2009 Merck Sharp & Dohme B.V., a subsidiary of Merck & Co., Inc.
All rights reserved.Revised: 03/2014
pcf-mk8415-ipt-1403r003
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 68 mg Carton
NDC 0052-0272-01
Organon
Manufactured for Organon USA Inc.
Roseland, NJ 07068
by N.V. Organon
Oss, The Netherlands
www.organon-usa.comThis product is intended to prevent
pregnancy. It does not protect against
HIV infection (AIDS) and other sexually
transmitted diseases.1 applicator containing 1 single rod subdermal implant
IMPLANON™
(etonogestrel implant)68 mg
For subdermal use onlyRx only
-
INGREDIENTS AND APPEARANCE
IMPLANON
etonogestrel implantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0052-0272 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength etonogestrel (UNII: 304GTH6RNH) (etonogestrel - UNII:304GTH6RNH) etonogestrel 68 mg Inactive Ingredients Ingredient Name Strength ETHYLENE-VINYL ACETATE COPOLYMER (15% VINYL ACETATE) (UNII: V9BQI51YUL) ETHYLENE-VINYL ACETATE COPOLYMER (28% VINYL ACETATE) (UNII: 8ILA5X28VS) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0052-0272-01 1 in 1 CARTON 09/06/2011 1 1 in 1 BLISTER PACK; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021529 09/06/2011 Labeler - Organon USA Inc. (078796541)
Trademark Results [IMPLANON]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 IMPLANON 78786272 3509988 Dead/Cancelled |
MERCK SHARP & DOHME B.V. 2006-01-06 |
 IMPLANON 76051947 not registered Dead/Abandoned |
N.V. Organon 2000-05-22 |
 IMPLANON 75017557 not registered Dead/Abandoned |
N.V. Organon 1995-11-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.