DIANEAL LOW CALCIUM WITH DEXTROSE- sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solution DIANEAL PD-2 WITH DEXTROSE- sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solution
DIANEAL PD-2 WITH DEXTROSE by
Drug Labeling and Warnings
DIANEAL PD-2 WITH DEXTROSE by is a Prescription medication manufactured, distributed, or labeled by Vantive US Healthcare LLC, Baxter Healthcare (Guangzhou) Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Health Care Provider Letter
-
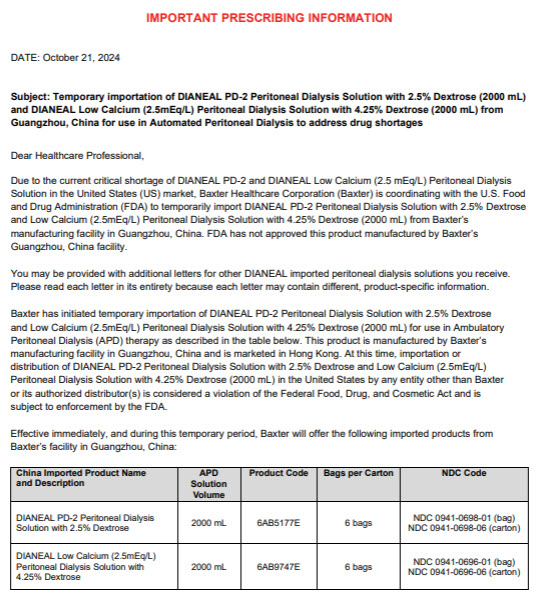
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
6AB5177E 2000ml
(APPROX 80ml EXCESS)
3000ml NOMINAL SIZE CONTAINERBaxterLogo
Dianeal® PD-2
Peritoneal Dialysis Solution
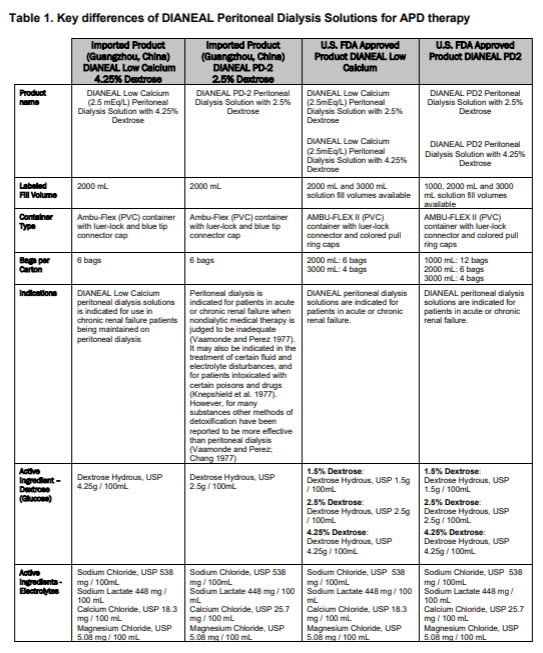
With 2.5% DextroseEACH 100ml CONTAINS2.5 g DEXTROSE HYDROUS USP 538 mg
SODIUM CHLORIDE USP 448 mg SODIUM LACTATE 25.7 mg CALCIUM
CHLORIDE USP 5.08 mg MAGNESIUM CHLORIDE USP pH5.2 (4.5 to 6.5)
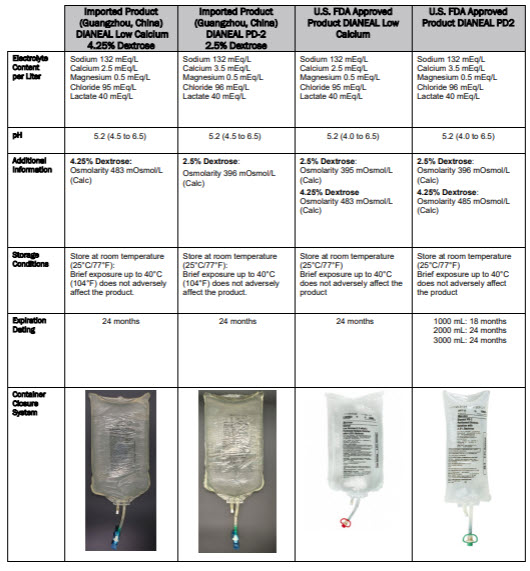
mEq/LSODIUM 132 CALCIUM 3.5 MAGNESIUM 0.5 CHLORIDE 96
LACTATE 40 OSMOLARITY396 mOsmol/L(CALC)
STERILE NON PYROGENICPOTASSIUM CHLORIDE TO BE ADDED ONLY UNDER THE
DIRECTION OF A PHYSICIANREAD PACKAGE INSERT FOR FULL INFORMATION
FOR INTRAPERITONEAL ADMINISTRATION ONLY
CAUTIONSSQUEEZE AND INSPECT INNER BAG WHICH
MAINTAINS PRODUCT STERILITY DISCARD IF LEAKS ARE FOUND
DO NOT USE UNLESS SOLUTION IS CLEAR
DISCARD UNUSED PORTIONSTORE UNIT IN MOISTURE BARRIER OVERWRAP AT ROOM
TEMPERATURE (UNDER 25°C) UNTIL READY TO USE
AVOID EXCESSIVE HEAT SEE INSERTAmbu-Flex TMIII CONTAINER PL-146 ® PLASTIC
MANUFACTURED BY
BAXTER HEALTHCARE (GUANGZHOU) CO LTD
GUANGZHOU CHINA
(AN AFFILIATE OF BAXTER WORLD TRADE INC USA)HK-42533
DIRECTIONSTO BE USED AS DIRECTED BY THE PHYSICIANPrescription Drug
Manufacturer Address:
Jiaoyuan Road, Dongji Industrial District,
GETDD, Guangzhou, P.R. China2.5 PD-2 WITH 2.5% DEXTROSE 2000mlX6
LOT G00000000 EXP JAN 00
6AB5177E
S/N 00006AB9747E 2000ml
(APPROX 80ml EXCESS)
3000ml NOMINAL SIZE CONTAINERBaxterLogo
Dianeal® Low Calcium(2.5mEq/L)
Peritoneal Dialysis Solution
With 4.25% DextroseEACH 100ml CONTAINS4.25 g DEXTROSE HYDROUS USP
538 mg SODIUM CHLORIDE USP 448 mg SODIUM LACTATE
18.3 mg CALCIUM CHLORIDE USP 5.08 mg MAGNESIUM
CHLORIDE USP pH5.2 (4.5 to 6.5)
mEq/LSODIUM 132 CALCIUM 2.5 MAGNESIUM 0.5
CHLORIDE 95 LACTATE 40
OSMOLARITY483 mOsmol/L(CALC)
STERILE NON PYROGENICPOTASSIUM CHLORIDE TO BE ADDED ONLY UNDER THE
DIRECTION OF A PHYSICIANWARNINGEXTENSIVE USE OF THIS SOLUTION DURING
ONE PERITONEAL DIALYSIS PROCEDURE CAN RESULT IN
SIGNIFICANT REMOVAL OF WATER FROM THE PATIENT
READ PACKAGE INSERT FOR FULL INFORMATION
FOR INTRAPERITONEAL ADMINISTRATION ONLY
CAUTIONSSQUEEZE AND INSPECT INNER BAG WHICH
MAINTAINS PRODUCT STERILITY DISCARD IF
LEAKS ARE FOUND
DO NOT USE UNLESS SOLUTION IS CLEAR
DISCARD UNUSED PORTION
STORE UNIT IN MOISTURE BARRIER OVERWRAP AT ROOM
TEMPERATURE (UNDER 25°C) UNTIL READY TO USE
AVOID EXCESSIVE HEAT SEE INSERTAmbu-Flex TMIII CONTAINER PL-146 ®PLASTIC
MANUFACTURED BY
BAXTER HEALTHCARE (GUANGZHOU) CO LTD
GUANGZHOU CHINA
(AN AFFILIATE OF BAXTER WORLD TRADE INC USA)HK-42532
DIRECTIONSTO BE USED AS DIRECTED BY THE PHYSICIANPrescription Drug
Manufacturer Address:
Jiaoyuan Road, Dongji Industrial District,
GETDD, Guangzhou, P.R. China4.25 LOW CALCIUM WITH 4.25% DEXTROSE 2000mlX6
LOT G00000000 EXP JAN 00
6AB9747E
S/N 0000 -
INGREDIENTS AND APPEARANCE
DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0941-0696 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 4.25 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.3 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0941-0696-06 6 in 1 CARTON 10/30/2024 12/31/2026 1 NDC: 0941-0696-01 2000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/30/2024 12/31/2026 DIANEAL PD-2 WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0941-0698 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 2.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 25.7 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0941-0698-06 6 in 1 CARTON 10/30/2024 12/31/2026 1 NDC: 0941-0698-01 2000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/30/2024 12/31/2026 Labeler - Vantive US Healthcare LLC (119181963) Establishment Name Address ID/FEI Business Operations Baxter Healthcare (Guangzhou) Co., Ltd 421040114 manufacture(0941-0696, 0941-0698) , analysis(0941-0696, 0941-0698) , label(0941-0696, 0941-0698) , pack(0941-0696, 0941-0698) , sterilize(0941-0696, 0941-0698)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.