BRIMONIDINE TARTRATE solution/ drops
Brimonidine Tartrate by
Drug Labeling and Warnings
Brimonidine Tartrate by is a Prescription medication manufactured, distributed, or labeled by Regimed Medical, Akorn, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Rx Only
-
DESCRIPTION
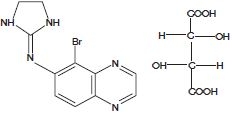
Brimonidine Tartrate Ophthalmic Solution, 0.2% is a relatively selective alpha-2 adrenergic agonist for ophthalmic use. The chemical name brimonidine tartrate is 5-bromo-6 (2-imidazolidinylideneamino) quinoxaline L-tartrate. It is a white to slightly yellowish powder. In solution, brimonidine tartrate has a clear, grennish-yellow color. It has a molecular weight of 442.24 as the tartrate salt and is water soluble (34 mg/mL). The molecular formula is C11H10BrN5C4H6O6; the structural formula is:
Brimonidine Tartrate Ophthalmic Solution, 0.2% is a sterile ophthalmic solution.
Each mL of Brimonidine Tartrate Solution contains:
ACTIVE: Brimonidine Tartrate 2 mg (equivalent to 1.32 mg as brimonidine free base).
PRESERVATIVE: Benzalkonium Chloride (0.05 mg)
INACTIVES: Citric Acid, Polyvinyl Alcohol, Sodium Citrate; Hydrochloric Acid and/or Sodium Hydroxide may be added to adjust pH (5.6 to 6.6), and Water for Injection.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Brimonidine Tartrate Ophthalmic Solution is an alpha adrenergic receptor agonist. It has a peak ocular hypotensive effect occurring at two hours post-dosing. Fluorophotometric studies in animals and humans suggest that brimonidine tartrate has a dual mechanism of action by reducing aqueous humor production and increasing uveoscleral outflow.
Pharmacokinetics
After ocular administration of a 0.2% solution, plasma concentrations peaked within 1 to 4 hours and declined with a systemic half-life of approximately 3 hours. In humans, systemic metabolism of brimonidine is extensive. It is metabolized primarily by the liver. Urinary excretion is the major route of elimination of the drug and its metabolites. Approximately 87% of an orally administered radioactive dose was eliminated within 120 hours, with 74% found in the urine.
Clinical Evaluations
Elevated IOP presents a major risk factor in glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss. Brimonidine Tartrate Ophthalmic Solution has the action of lowering intraocular pressure with minimal effect on cardiovascular and pulmonary parameters.
In comparative clinical studies with timolol 0.5%, lasting up to one year, the IOP lowering effect of Brimonidine Tartrate Ophthalmic Solution was approximately 4-6 mm Hg compared with approximately 6 mm Hg for timolol. In these studies, both patient groups were dosed BID; however, due to the duration of action of Brimonidine Tartrate Ophthalmic Solution, it is recommended that Brimonidine Tartrate Ophthalmic Solution be dosed TID. Eight percent of subjects were discontinued from studies due to inadequately controlled intraocular pressure, which in 30% of these patients occurred during the first month of therapy. Approximately 20% were discontinued due to adverse experiences.
-
INDICATIONS AND USAGE
Brimonidine Tartrate Ophthalmic Solution is indicated for lowering intraocular pressure in patients with open-angle glaucoma or ocular hypertension. The IOP lowering efficacy of Brimonidine Tartrate Ophthalmic Solution diminishes over time in some patients. This loss of effect appears with a variable time of onset in each patient and should be closely monitored.
- CONTRAINDICATIONS
-
PRECAUTIONS
General
Although Brimonidine Tartrate Ophthalmic Solution had minimal effect on blood pressure of patients in clinical studies, caution should be exercised in treating patients with severe cardiovascular disease.
Brimonidine Tartrate Ophthalmic Solution has not been studied in patients with hepatic or renal impairment; caution should be used in treating such patients.
Brimonidine Tartrate Ophthalmic Solution should be used with caution in patients with depression, cerebral or coronary insufficiency, Raynaud's phenomenon, orthostatic hypotension or thromboangiitis obliterans.
During the studies there was a loss of effect in some patients. The lOP-lowering efficacy observed with Brimonidine Tartrate Ophthalmic Solution during the first month of therapy may not always reflect the long-term level of IOP reduction. Patients prescribed lOP-lowering medication should be routinely monitored for IOP.
Information for Patients:
The preservative in Brimonidine Tartrate Ophthalmic Solution, benzalkonium chloride, may be absorbed by soft contact lenses. Patients wearing soft contact lenses should be instructed to wait at least 15 minutes after instilling Brimonidine Tartrate Ophthalmic Solution to insert soft contact lenses.
As with other drugs in this class, Brimonidine Tartrate Ophthalmic Solution may cause fatigue and/or drowsiness in some patients. Patients who engage in hazardous activities should be cautioned of the potential for a decrease in mental alertness.
Drug Interactions:
Although specific drug interaction studies have not been conducted with Brimonidine Tartrate Ophthalmic Solution, the possibility of an additive or potentiating effect with CNS depressants (alcohol, barbiturates, opiates, sedatives, or anesthetics) should be considered. Alpha-agonists, as a class, may reduce pulse and blood pressure. Caution in using concomitant drugs such as beta-blockers (ophthalmic and systemic): antihypertensives and/or cardiac glycosides is advised.
Tricyclic antidepressants have been reported to blunt the hypotensive effect of systemic clonidine. It is not known whether the concurrent use of these agents with Brimonidine Tartrate Ophthalmic Solution in humans can lead to resulting interference with I0P lowering effect. No data on the level of circulating catecholamines after Brimonidine Tartrate Ophthalmic Solution is instilled are available. Caution, however, is advised in patients taking tricyclic antidepressants which can affect the metabolism and uptake of circulating amines.
Carcinogenesis, mutagenesis, impairment of fertility:
No compound-related carcinogenic effects were observed in either mice or rats following a 21-month and 24-month study, respectively. In these studies, dietary administration of brimonidine tartrate at doses up to 2.5 mg/kg/day in mice and 1.0 mg/kg/day in rats achieved - 77 and 118 times, respectively, the plasma drug concentration estimated in humans treated with one drop of Brimonidine Tartrate Ophthalmic Solution into both eyes 3 times per day.
Brimonidine tartrate was not mutagenic or cytogenic in a series of in vitro and in vivo studies including the Ames test, chromosomal aberration assay in Chinese Hamster Ovary (CHO) cells, a host-mediated assay and cytogenic studies in mice and dominant lethal assay.
Reproductive studies performed in rats with oral doses of 0.66 mg base/kg revealed no evidence of harm to the fetus due to Brimonidine Tartrate Ophthalmic Solution.
Pregnancy: Teratogenic Effects: Pregnancy Category B.
Reproduction studies performed in rats with oral doses of 0.66 mg base/kg revealed no evidence of harm to the fetus due to Brimonidine Tartrate Ophthalmic Solution. Dosing at this level produced 100 times the plasma drug concentration level seen in humans following multiple ophthalmic doses.
There are no adequate and well-controlled studies in pregnant women. In animals studies, brimonidine crossed the placenta and entered into the fetal circulation to a limited extent. Brimonidine Tartrate Ophthalmic Solution should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus.
Nursing Mothers:
It is not known whether Brimonidine Tartrate Ophthalmic Solution is excreted in human milk, although in animal studies, brimonidine tartrate has been shown to be excreted in breast milk. A decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use:
In a well-controlled clinical study conducted in pediatric glaucoma patients (ages 2 to 7 years) the most commonly observed adverse events with brimonidine tartrate ophthalmic solution 0.2% dosed three times daily were somnolence (50% - 83% in patients ages 2 to 6 years) and decreased alertness. In pediatric patients 7 years of age or older (>20kg), somnolence appears to occur less frequently (25%). The most commonly observed adverse event was somnolence. Approximately 16% of patients on brimonidine tartrate ophthalmic solution discontinued from the study due to somnolence.
The safety and effectiveness of Brimonidine Tartrate Ophthalmic Solution have not been studied in pediatric patients below the age of 2 years. Brimonidine Tartrate Ophthalmic Solution is not recommended under the age of 2 years (Also refer to ADVERSE REACTIONS).
Geriatric Use:
No overall differences in safety or effectiveness have been observed between elderly and other adult patients.
-
ADVERSE REACTIONS
Adverse events occurring in approximately 10-30% of the subjects, in descending order of incidence, included oral dryness, ocular hyperemia: burning and stinging, headache, blurring, foreign body sensation, fatigue/drowsiness, conjunctival follicles, ocular allergic reactions, and ocular pruritus.
Events occurring in approximately 3-9% of the subjects, in descending order included corneal staining/erosion, photophobia, eyelid erythema: ocular ache/pain, ocular dryness, tearing, upper respiratory symptoms, eyelid edema, conjunctival edema, dizziness, blepharitis, ocular irritation gastrointestinal symptoms, asthenia, conjunctival blanching, abnormal vision and muscular pain.
The following adverse reactions were reported in less than 3% of the patients: lid crusting, conjunctival hemorrhage, abnormal taste, insomnia: conjunctival discharge, depression, hypertension, anxiety, palpitations/arrhythmias, nasal dryness and syncope.
The following events have been identified during post-marketing use of Brimonidine Tartrate Ophthalmic Solution in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The events, which have been chosen for inclusion due to either their seriousness, frequency of reporting, causal connection to Brimonidine Tartrate Ophthalmic Solution, or a combination of these factors, include: bradycardia; hypotension; iritis; miosis; skin reactions (including erythema, eyelid purities, rash, and vasodilation); and tachycardia. Apnea, bradycardia, hypotension, hypothermia, hypotonia, and somnolence have been reported in infants receiving Brimonidine Tartrate Ophthalmic Solution.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
The recommended dose is one drop of Brimonidine Tartrate Ophthalmic Solution in the affected eye(s) three times daily, approximately 8 hours apart.
Brimonidine Tartrate Ophthalmic Solution may be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure. If more than one topical ophthalmic product is being used, the products should be administered at least 5 minutes apart.
- HOW SUPPLIED
- WARNING
- STORAGE
-
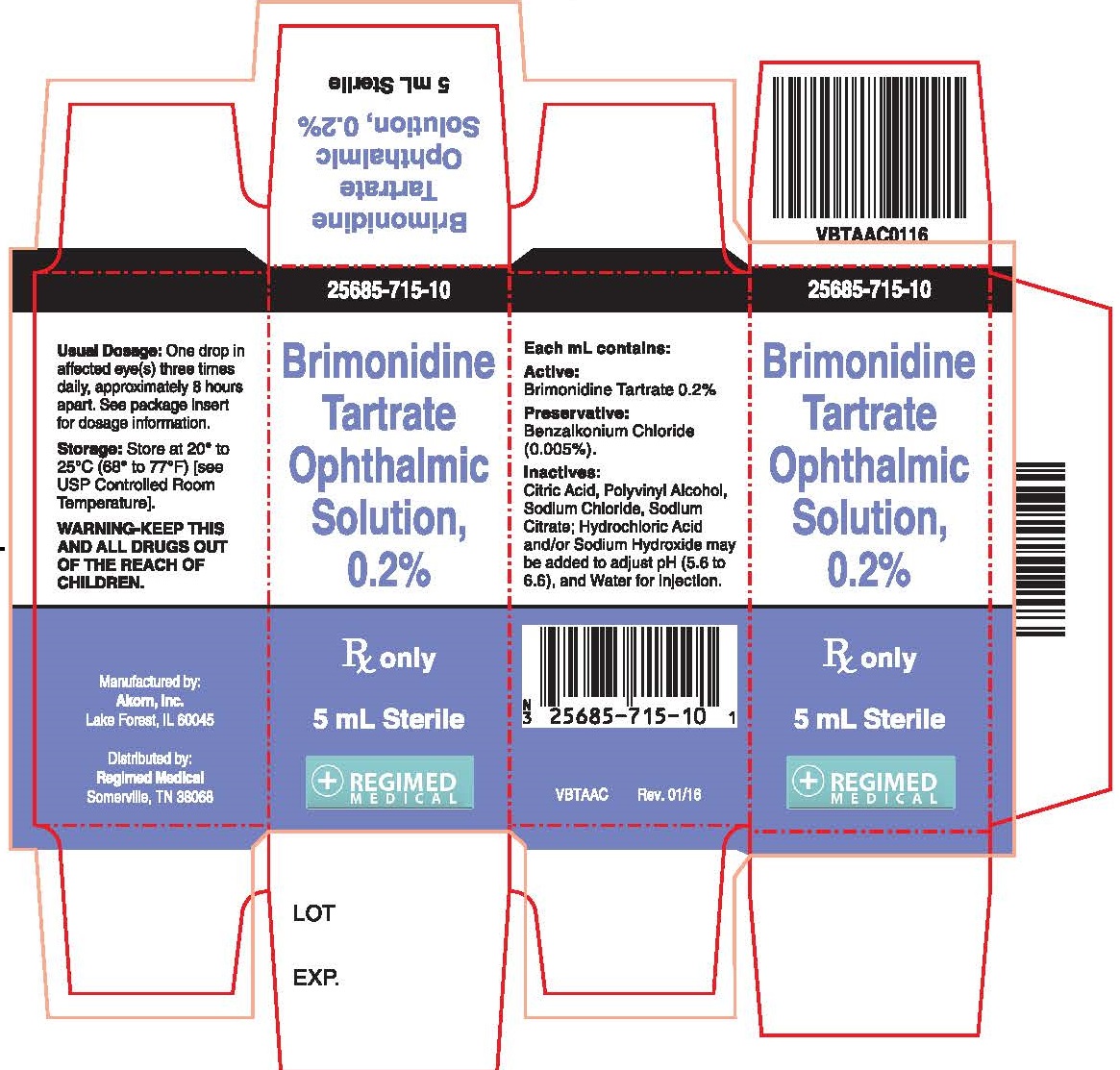
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 25685-715-10
5 mL
Brimonidine
Tartrate
Ophthalmic
Solutions, 0.2%
Rx Only Sterile
Mfd. by Akorn, Inc.
Distributed by: Regimed Medical
VBTAAL Rev. 01/16

-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 25685-715-10
Brimonidine
Tartrate
Ophthalmic
Solutions, 0.2%
Rx Only
5 mL Sterile
Manufactured by:
Akorn, Inc.
Lake Forest, IL 60045
Distributed by:
Regimed Medical
Somerville, TN 38068
VBTAAC Rev. 01/16
-
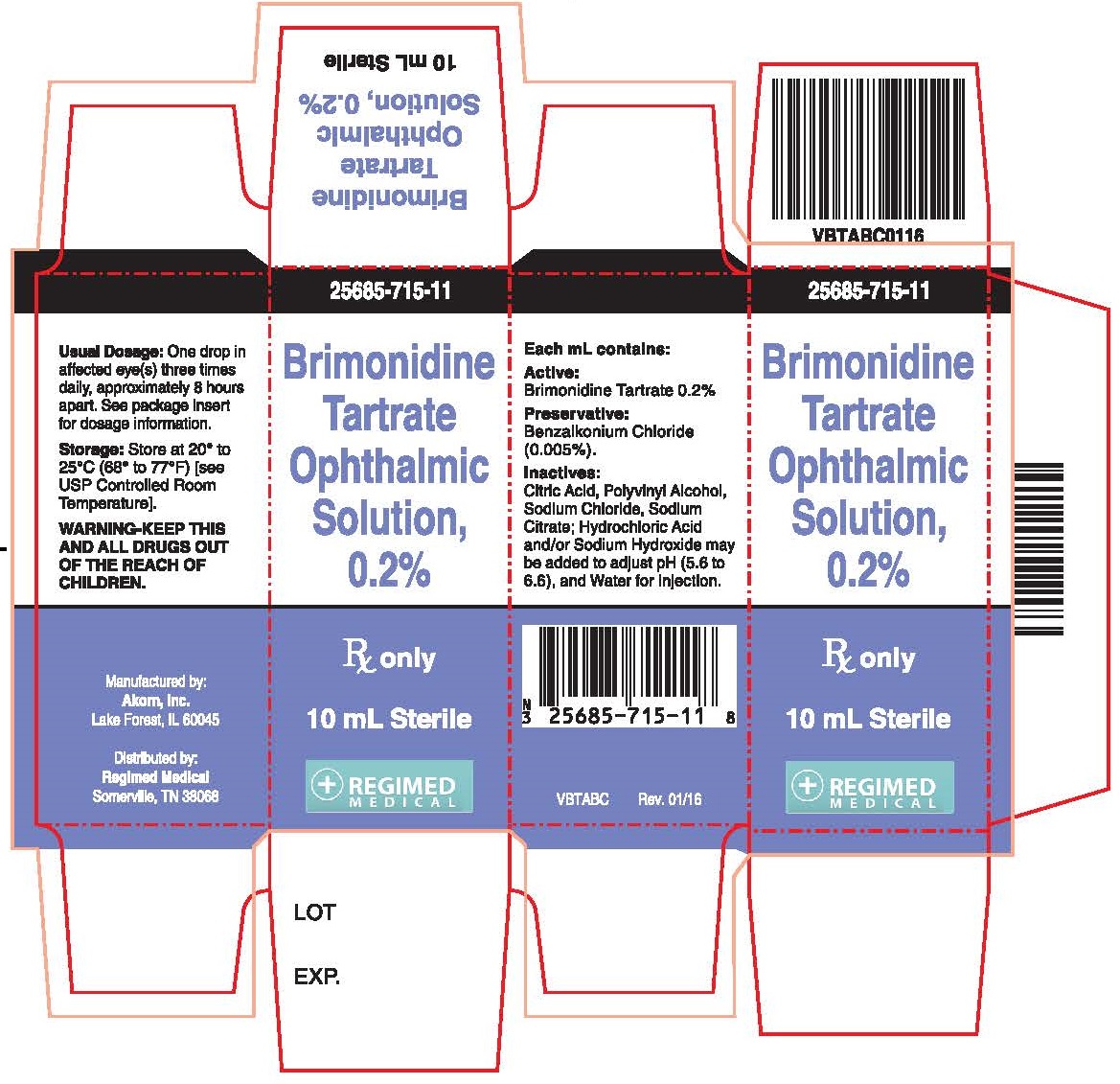
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 25685-715-11
10 mL
Brimonidine
Tartrate
Ophthalmic
Solutions, 0.2%
Rx Only Sterile
Mfd. by Akorn, Inc.
Distributed by: Regimed Medical
VBTABL Rev. 01/16

-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 25685-715-11
Brimonidine
Tartrate
Ophthalmic
Solutions, 0.2%
Rx Only
10 mL Sterile
Manufactured by:
Akorn, Inc.
Lake Forest, IL 60045
Distributed by:
Regimed Medical
Somerville, TN 38068
VBTABC Rev. 01/16
-
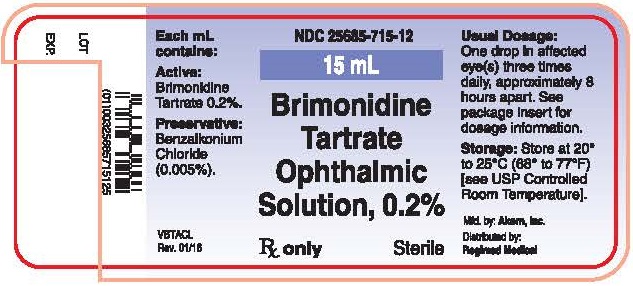
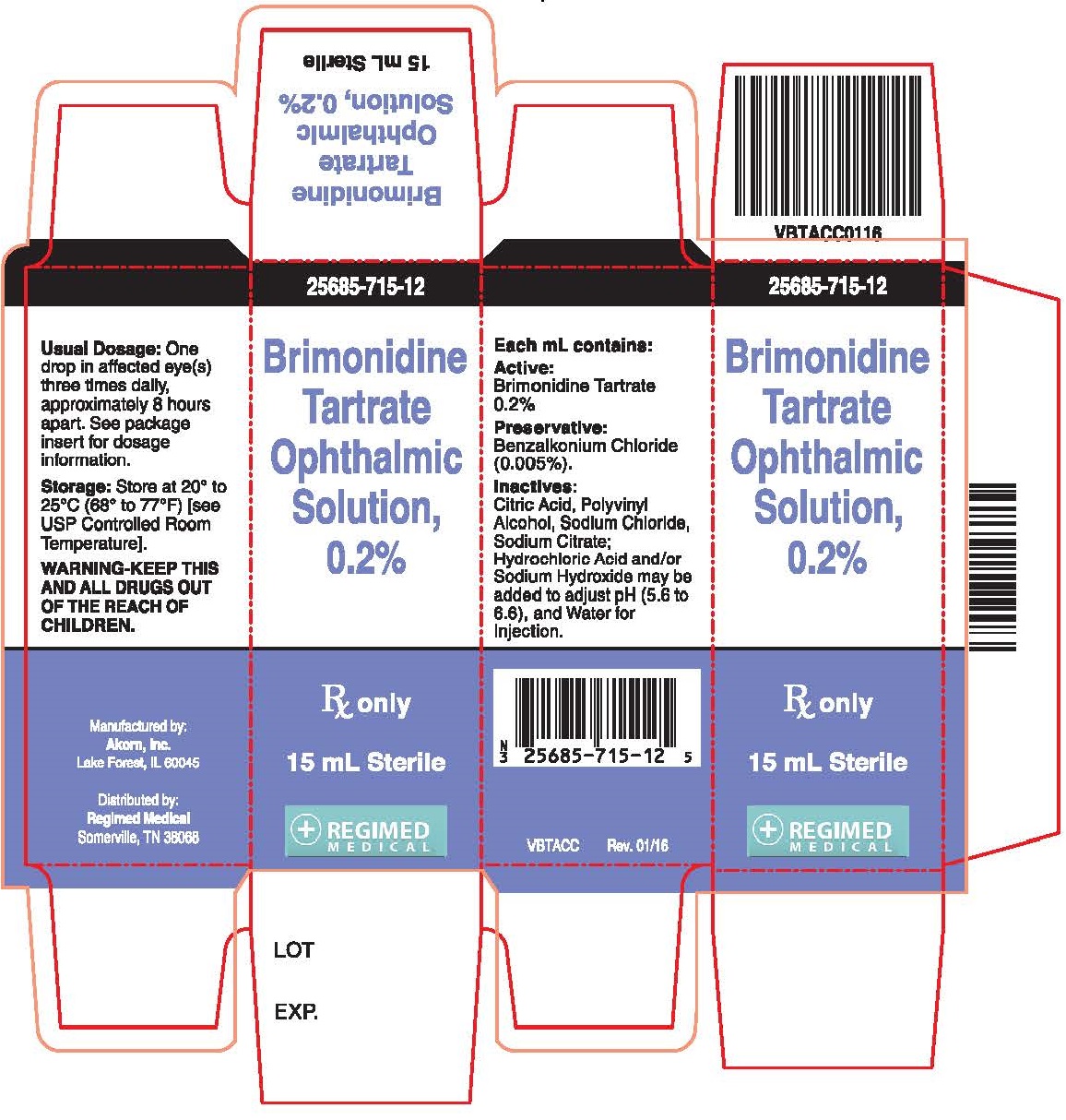
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 25685-715-12
15 mL
Brimonidine
Tartrate
Ophthalmic
Solutions, 0.2%
Rx Only Sterile
Mfd. by Akorn, Inc.
Distributed by: Regimed Medical
VBTACL Rev. 01/16
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 25685-715-12
Brimonidine
Tartrate
Ophthalmic
Solutions, 0.2%
Rx Only
15 mL Sterile
Manufactured by:
Akorn, Inc.
Lake Forest, IL 60045
Distributed by:
Regimed Medical
Somerville, TN 38068
VBTACC Rev. 01/16
-
INGREDIENTS AND APPEARANCE
BRIMONIDINE TARTRATE
brimonidine tartrate solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 25685-715 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRIMONIDINE TARTRATE (UNII: 4S9CL2DY2H) (BRIMONIDINE - UNII:E6GNX3HHTE) BRIMONIDINE 2.0 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POLYVINYL ALCOHOL (UNII: 532B59J990) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CITRATE (UNII: 1Q73Q2JULR) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25685-715-10 1 in 1 CARTON 1 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC: 25685-715-11 1 in 1 CARTON 2 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 3 NDC: 25685-715-12 1 in 1 CARTON 3 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076439 03/03/2016 Labeler - Regimed Medical (809916570) Establishment Name Address ID/FEI Business Operations Akorn, Inc. 063434679 manufacture(25685-715)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
