CROMOLYN SODIUM solution/ drops
Cromolyn Sodium by
Drug Labeling and Warnings
Cromolyn Sodium by is a Prescription medication manufactured, distributed, or labeled by NuCare Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Cromolyn Sodium Ophthalmic Solution, USP 4% is a clear, colorless, sterile solution for topical ophthalmic use.
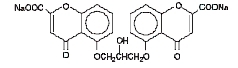
Cromolyn sodium is represented by the following structural formula:
C 23H 14Na 2O 11 Mol. Wt. 512.34
Chemical Name: Disodium 5,5'- [(2-hydroxytrimethylene)dioxy]bis[4-oxo-4H-1-benzopyran-2-carboxylate]
Pharmacologic Category: Mast cell stabilizer.
Each mL contains: Active: Cromolyn Sodium 40 mg (4%); Inactives: Edetate Disodium 0.1% and Purified Water, USP. Hydrochloric Acid and/or Sodium Hydroxide may be added to adjust pH (4.0 - 7.0). Preservative: Benzalkonium Chloride 0.01%.
-
CLINICAL PHARMACOLOGY
In vitro and in vivo animal studies have shown that cromolyn sodium inhibits the degranulation of sensitized mast cells which occurs after exposure to specific antigens. Cromolyn sodium acts by inhibiting the release of histamine and SRS-A (slow-reacting substance of anaphylaxis) from the mast cell.
Another activity demonstrated in vitro is the capacity of cromolyn sodium to inhibit the degranulation of non-sensitized rat mast cells by phospholipase A and the subsequent release of chemical mediators. Another study showed that cromolyn sodium did not inhibit the enzymatic activity of released phospholipase A on its specific substrate.
Cromolyn sodium has no intrinsic vasoconstrictor, antihistamine, or anti-inflammatory activity.
Cromolyn sodium is poorly absorbed. When multiple doses of cromolyn sodium ophthalmic solution are instilled into normal rabbit eyes, less than 0.07% of the administered dose of cromolyn sodium is absorbed into the systemic circulation (presumably by way of the eye, nasal passages, buccal cavity and gastrointestinal tract). Trace amounts (less than 0.01%) of the cromolyn sodium dose penetrate into the aqueous humor and clearance from this chamber is virtually complete within 24 hours after treatment is stopped.
In normal volunteers, analysis of drug excretion indicates that approximately 0.03% of cromolyn sodium is absorbed following administration to the eye.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General:
Patients may experience a transient stinging or burning sensation following application of Cromolyn Sodium Ophthalmic Solution.
The recommended frequency of administration should not be exceeded ( see DOSAGE AND ADMINISTRATION).
Information for Patients:
Patients should be advised to follow the patient instructions listed on the Information for Patients sheet.
Users of contact lenses should refrain from wearing lenses while exhibiting the signs and symptoms of vernal keratoconjunctivitis, vernal conjunctivitis, or vernal keratitis. Do not wear contact lenses during treatment with Cromolyn Sodium Ophthalmic Solution.
Carcinogenesis, Mutagenesis, and Impairment of Fertility:
Long-term studies of cromolyn sodium in mice (12 months intraperitoneal administration at doses up to 150 mg/kg three days per week): hamsters (intraperitoneal administration at doses up to 52.6 mg/kg three days per week for 15 weeks followed by 17.5 mg/kg three days per week for 37 weeks), and rats (18 months subcutaneous administration at doses up to 75 mg/kg six days per week) showed no neoplastic effects. The average daily maximum dose levels administered in these studies were 192.9 mg/m 2 for mice, 47.2 mg/m 2 for hamsters and 385.8 mg/m 2 for rats. These doses correspond to approximately 6.8, 1.7, and 14 times the maximum daily human dose of 28 mg/m 2.
Cromolyn sodium showed no mutagenic potential in the Ames Salmonella/microsome plate assays, mitotic gene conversion in Saccharomyces cerevisiae and in an in vitro cytogenetic study in human peripheral lymphocytes.
No evidence of impaired fertility was shown in laboratory reproduction studies conducted subcutaneously in rats at the highest doses tested, 175 mg/kg/day (1050 mg/m 2) in males and 100 mg/kg/day (600 mg/m 2) in females. These doses are approximately 37 and 21 times the maximum daily human dose, respectively, based on mg/m 2.
Teratogenic effects:
Pregnancy Category B. Reproduction studies with cromolyn sodium administered subcutaneously to pregnant mice and rats at maximum daily doses of 540 mg/kg (1620 mg/m 2) and 164 mg/kg (984 mg/m 2), respectively, and intravenously to rabbits at a maximum daily dose of 485 mg/kg (5820 mg/m 2) produced no evidence of fetal malformation. These doses represent approximately 57, 35, and 205 times the maximum daily human dose, respectively, on a mg/m 2 basis. Adverse fetal effects (increased resorption and decreased fetal weight) were noted only at the very high parenteral doses that produced maternal toxicity. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
-
ADVERSE REACTIONS
The most frequently reported adverse reaction attributed to the use of cromolyn sodium ophthalmic solution, on the basis of reoccurrence following readministration, is transient ocular stinging or burning upon instillation.
The following adverse reactions have been reported as infrequent events. It is unclear whether they are attributed to the drug:
Conjunctival injection; watery eyes; itchy eyes; dryness around the eye; puffy eyes; eye irritation; and styes.
Immediate hypersensitivity reactions have been reported rarely and include dyspnea, edema, and rash.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch + Lomb, a division of Valeant Pharmaceuticals North America LLC, at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
DOSAGE AND ADMINISTRATION
The dose is 1 or 2 drops in each eye 4 to 6 times a day at regular intervals.
One drop contains approximately 1.6 mg cromolyn sodium.
Patients should be advised that the effect of Cromolyn Sodium Ophthalmic Solution therapy is dependent upon its administration at regular intervals, as directed.
Symptomatic response to therapy (decreased itching, tearing, redness, and discharge) is usually evident within a few days, but longer treatment for up to six weeks is sometimes required. Once symptomatic improvement has been established, therapy should be continued for as long as needed to sustain improvement.
If required, corticosteroids may be used concomitantly with Cromolyn Sodium Ophthalmic Solution.
FOR OPHTHALMIC USE ONLY
-
HOW SUPPLIED
Cromolyn Sodium Ophthalmic Solution, USP 4% is supplied in a plastic bottle individually cartoned with a white cap and controlled drop tip in the following sizes:
10 mL bottle NDC: 68071-3231-1
DO NOT USE IF IMPRINTED NECKBAND IS NOT INTACT.
Storage:
Store between 15°-25°C (59°-77°F). Protect from light – store in original carton. Keep tightly closed. Replace cap immediately after use.
KEEP OUT OF REACH OF CHILDREN.
Revised August 2016
Bausch + Lomb, a division of Valeant Pharmaceuticals North America LLC
Bridgewater, NJ 08807 USA
©Bausch & Lomb Incorporated9087003 (Folded)
9087103 (Flat) -
PATIENT PACKAGE INSERT
PHARMACIST — DETACH HERE AND GIVE INSTRUCTIONS TO PATIENTS
Information for the Patient
Cromolyn Sodium Ophthalmic Solution, USP 4% (STERILE )
It is important to use Cromolyn Sodium Ophthalmic Solution, USP 4% regularly, as directed by your physician.
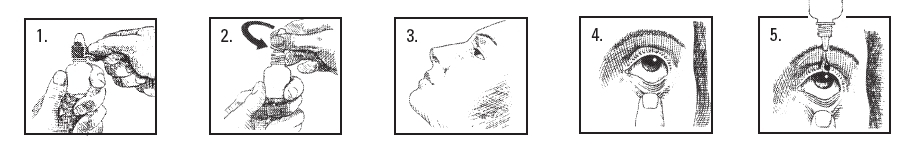
1. Thoroughly wash your hands.
2. Remove safety seal (Figure 1).
3. Remove cap (Figure 2).
4. Sit or stand comfortably, with your head tilted back (Figure 3).
5. Open eyes, look up, and draw the lower lid of your eye down gently with your index finger (Figure 4).
6. Hold the Cromolyn Sodium Ophthalmic Solution, USP 4% bottle upside down. Place dropper tip as close as possible to the lower eyelid and gently squeeze out the prescribed number of drops (Figure 5).
7. Do not touch the eye or eyelid with the dropper tip.
8. Blink a few times to make sure the eye is covered with the solution.
9. Close your eye and remove any excess solution with a clean tissue.
10. Repeat process in the other eye.
SPECIAL TIPS
1. Avoid placing Cromolyn Sodium Ophthalmic Solution, USP 4% solution directly on the cornea (the area just over the pupil), because it is especially sensitive. You will find the administration of eye drops more comfortable if you place the drops just inside the lower eyelid, as shown in Figure 5 on the previous page.
2. To avoid contamination of the solution, do not touch dropper tip to the eye, fingers or any other surface. Replace cap after use. It is recommended that any remaining contents be discarded after the treatment period prescribed by your physician.
3. Store between 15°-25°C (59°-77°F). Protect from light – store in original carton. Keep tightly closed. Replace cap immediately after use.
4. Keep tightly closed and out of the reach of children.
5. Do not use with any other ocular medication unless directed by your physician. Do not wear contact lenses during treatment with Cromolyn Sodium Ophthalmic Solution, USP 4%.
Bausch + Lomb, a division of Valeant Pharmaceuticals North America LLC
Bridgewater, NJ 08807 USA
©Bausch & Lomb Incorporated9087003 (Folded)
9087103 (Flat) - Principal Display Panel – Cromolyn Carton Label -10mL
-
INGREDIENTS AND APPEARANCE
CROMOLYN SODIUM
cromolyn sodium solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68071-3231(NDC: 24208-961) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CROMOLYN SODIUM (UNII: Q2WXR1I0PK) (CROMOLYN - UNII:Y0TK0FS77W) CROMOLYN SODIUM 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68071-3231-1 10 mL in 1 BOX; Type 0: Not a Combination Product 06/12/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074443 01/30/1995 Labeler - NuCare Pharmaceuticals, Inc. (010632300) Establishment Name Address ID/FEI Business Operations NuCare Pharmaceuticals, Inc. 010632300 relabel(68071-3231)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.