ANTIBACTERIAL HAND SANITIZER- ethyl alcohol gel
Antibacterial Hand Sanitizer by
Drug Labeling and Warnings
Antibacterial Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Zhejiang Ayan Biotech Co.,Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
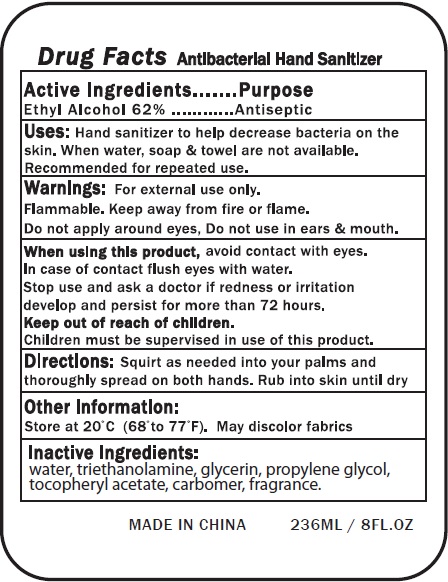
- Drug Facts
- Active Ingredients
- Uses:
- Warnings:
- Directions:
- Other Information:
- Inactive Ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
ANTIBACTERIAL HAND SANITIZER
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70412-112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 620 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70412-112-36 1 in 1 BOX 01/18/2016 1 236 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/18/2016 Labeler - Zhejiang Ayan Biotech Co.,Ltd. (544377996)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.