SORILUX- calcipotriene aerosol, foam
SORILUX by
Drug Labeling and Warnings
SORILUX by is a Prescription medication manufactured, distributed, or labeled by Mayne Pharma, DPT Laboratories, Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SORILUX safely and effectively. See full prescribing information for SORILUX.
SORILUX (calcipotriene) foam, for topical use
Initial U.S. Approval: 1993RECENT MAJOR CHANGES
Indications and Usage (1) 11/2019 INDICATIONS AND USAGE
SORILUX® Foam is a vitamin D analog indicated for the topical treatment of plaque psoriasis of the scalp and body in adults and pediatric patients 4 years of age and older. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
0.005%, foam. (3)
CONTRAINDICATIONS
- Do not use in patients with known hypercalcemia. (4)
WARNINGS AND PRECAUTIONS
- Flammability: Contents are flammable. Instruct the patient the avoid fire, flame, and smoking during and immediately following application. (5.1)
- Effects on Calcium Metabolism: If elevation of serum calcium occurs, instruct patients to discontinue treatment until normal calcium levels are restored. (5.2)
ADVERSE REACTIONS
Adverse reactions reported in ≥ 1% of subjects treated with SORILUX Foam and at a higher incidence than subjects treated with vehicle were application site erythema and application site pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma at 1-844-825-8500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Flammability
5.2 Effects on Calcium Metabolism
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
SORILUX Foam was studied in four vehicle-controlled trials. A total of 1094 adult subjects with plaque psoriasis, including 654 exposed to SORILUX Foam, were treated twice daily for 8 weeks. Adverse reactions reported in ≥1% of subjects treated with SORILUX Foam and at a higher incidence than subjects treated with vehicle were application site erythema (2%) and application site pain (3%). The incidence of these adverse reactions was similar between the body and scalp.
In an open-label study, 19 pediatric subjects age 12 to less than 17 years applied SORILUX Foam twice daily for 14 days and once on Day 15. Adverse reactions included application site pain, application site pruritus and pruritus [see Clinical Pharmacology (12.2 and 12.3) and Pediatric Use (8.4)].
In an open-label study, 36 pediatric subjects age 4 to less than 12 years applied SORILUX Foam twice daily for up to 8 weeks. Adverse reactions included application site pain and contact dermatitis [see Clinical Pharmacology (12.2 and 12.3) and Pediatric Use (8.4)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of SORILUX Foam. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous: application site vesicles
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Although there are no available data on the drug- associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes in pregnant women exposed to SORILUX Foam, systemic exposure to calcipotriene is likely to be low [see Clinical Pharmacology (12.2, 12.3)].
In animal reproduction studies, oral administration of calcipotriene to pregnant rats and rabbits during the period of organogenesis resulted in an increased incidence of minor skeletal abnormalities, including enlarged fontanelles and extra ribs in rats and an increased incidence of minor skeletal abnormalities, including incomplete ossification of pubic bones and forelimb phalanges in rabbits (see Data). The available data do not allow the calculation of relevant comparisons between the systemic exposure of calcipotriene observed in animal studies to the systemic exposure that would be expected in humans after topical use of SORILUX Foam.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Embryofetal development studies were conducted with calcipotriene after oral administration in rats and rabbits. Pregnant rats received daily oral administration of calcipotriene during the period of organogenesis. Fetuses from dams dosed with 54 mcg/kg/day (318 mcg/m2/day) exhibited a significantly increased incidence of minor skeletal abnormalities consisting primarily of enlarged fontanelles and extra ribs. The enlarged fontanelles are most likely due to calcipotriene's effect upon calcium metabolism. There were no effects on the incidence of major malformations in fetuses.
Pregnant rabbits received daily oral administration of calcipotriene during the period of organogenesis. Increased rabbit maternal and fetal toxicity was noted at 12 mcg/kg/day (132 mcg/m2/day). Fetuses from does dosed with 36 mcg/kg/day (396 mcg/m2/day) exhibited a significantly increased incidence of minor skeletal abnormalities including incomplete ossification of pubic bones and forelimb phalanges. There were no effects on the incidence of major malformations in fetuses.
8.2 Lactation
Risk Summary
There are no data on the presence of topically administered calcipotriene in human or animal milk, the effects on the breastfed infant, or the effects on milk production. After topical administration of SORILUX Foam, concentrations of calcipotriene in plasma are low, and therefore, concentrations in human milk are likely to be low [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for and any potential adverse effects on the breastfed child from SORILUX Foam or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of SORILUX Foam have been established in pediatric patients age 4 years and older for topical treatment of plaque psoriasis of the scalp and body.
Use of SORILUX Foam in this age group is supported by two adequate and well controlled 8-week trials in adults and adolescents 12 years of age and older, with additional data from a 15-day open-label safety and pharmacokinetics (PK) study conducted in 19 subjects 12 to less than 17 years of age; and an 8-week open-label safety and PK study in 36 subjects 4 to 11 years of age with psoriasis. Data from 19 subjects aged 12 to less than 17 years and 18 subjects aged 5 to 11 years showed no significant effects on indices of calcium metabolism. Systemic concentrations of calcipotriene were not quantifiable in the two studies in subjects aged 7 years to less than 17 years. [see Clinical Studies (14), Clinical Pharmacology (12.2, 12.3) and Adverse Reactions (6.1)].
The safety and effectiveness of SORILUX Foam in pediatric patients less than 4 years of age have not been established.
8.5 Geriatric Use
Clinical trials of SORILUX Foam did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
10 OVERDOSAGE
Topically applied calcipotriene can be absorbed in sufficient amounts to produce systemic effects. Elevated serum calcium has been observed with use of topical calcipotriene [See Warnings and Precautions (5.2)].
-
11 DESCRIPTION
SORILUX Foam contains the compound calcipotriene, a synthetic vitamin D3 analog, in an aqueous-based emulsion foam vehicle for topical dermatologic use.
Chemically, calcipotriene is (5Z,7E,22E,24S)-24-cyclopropyl-9,10-secochola-5,7,10(19), 22-tetraene- 1α,3β,24-triol. The structural formula is represented below:
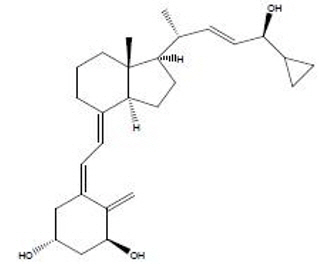
Molecular Formula: C27H40O3 Molecular Weight: 412.6
Calcipotriene is a white or off-white crystalline substance. SORILUX Foam contains calcipotriene 50 mcg/g in an aqueous-based emulsion foam vehicle consisting of cetyl alcohol, dibasic sodium phosphate, dl-α-tocopherol, edetate disodium, isopropyl myristate, light mineral oil, polyoxyl 20 cetostearyl ether, propylene glycol, purified water, stearyl alcohol, and white petrolatum. SORILUX Foam is dispensed from an aluminum can pressurized with a hydrocarbon (propane/n-butane/isobutane) propellant.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Calcipotriene is a synthetic vitamin D3 analog that has a similar receptor binding affinity as natural vitamin D3. However, the exact mechanism of action contributing to the clinical efficacy in the treatment of psoriasis is unknown.
12.2 Pharmacodynamics
Effects on Calcium Metabolism
In 19 subjects with psoriasis aged 12 to less than 17 years treated with SORILUX Foam two times a day for 15 days, there was no significant effect on indices of calcium metabolism including serum albumin adjusted calcium, urine calcium/creatinine ratio, iPTH, alkaline phosphatase, magnesium and phosphorus.
The effects of SORILUX Foam, 0.005%, on calcium metabolism were evaluated in 18 pediatric subjects 5 to 11 years of age receiving SORILUX Foam administered twice daily for a minimum of 14 days (mean: 56 days, range: 14 to 69 days). These subjects had plaque psoriasis involving an average of 10% of the body surface area (BSA) (range: 0.5%-36.5%). There was no relationship between urinary calcium/creatinine ratio (comparing Week 2 to Baseline) and % BSA treated.
12.3 Pharmacokinetics
The systemic absorption of calcipotriene in subjects with psoriasis of the body was evaluated at steady state following application of either SORILUX Foam or calcipotriene ointment to a body surface area of 5% to 10%. In the SORILUX Foam treatment group, 15 out of 16 subjects had calcipotriene plasma concentrations below the limit of quantitation (10 pg/mL), while in the calcipotriene ointment treated group, 5 out of 16 subjects had measurable calcipotriene plasma concentrations at various time points. All measurable plasma calcipotriene concentrations were below 25 pg/mL.
The pharmacokinetics of SORILUX Foam, 0.005% was investigated when applied topically for 15 days to 17 subjects aged 12 to less than 17 years with moderate plaque psoriasis involving a mean BSA of 24%, excluding face and scalp, and a mean scalp involvement of 43%. Systemic concentration of calcipotriene were not quantifiable in any of the subjects (limit of quantification = 10 pg/mL).
In 11 pediatric subjects 7 to 11 years of age with plaque psoriasis involving a mean BSA of 10%, plasma concentration of calcipotriene was measured following 2 weeks of twice daily administration of SORILUX Foam. No subject had quantifiable calcipotriene plasma concentration (limit of quantification = 10 pg/mL).
The systemic disposition of calcipotriene is expected to be similar to that of the naturally occurring vitamin D. Absorbed calcipotriene is known to be converted to inactive metabolites within 24 hours of application and the metabolism occurs via a similar pathway to the natural hormone.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Calcipotriene topically administered to mice for up to 24 months at dose levels of 3, 10, or 30 mcg/kg/day (corresponding to 9, 30, or 90 mcg /m2/day) showed no significant changes in tumor incidence when compared with controls.
The genotoxic potential of calcipotriene was evaluated in an Ames assay, a mouse lymphoma TK locus assay, a human lymphocyte chromosome aberration assay, and a mouse micronucleus assay. All assay results were negative.
Studies in rats at oral doses up to 54 mcg /kg/day (318 mcg /m2/day) of calcipotriene indicated no impairment of fertility or general reproductive performance.
-
14 CLINICAL STUDIES
In two multi-center, randomized, double-blind, vehicle-controlled clinical trials a total of 659 subjects with psoriasis were randomized 2:1 to SORILUX Foam or vehicle; subjects applied the assigned treatment twice daily for 8 weeks. Baseline disease severity was graded using a 5-point Investigator Static Global Assessment scale (ISGA), on which subjects scored either "mild" or "moderate" as shown in Table 1.
Table 1. Investigator Static Global Assessment (ISGA) Scale for Body Disease Severity Grade Definition Clear 0 No evidence of scaling, erythema, or plaque thickness Almost clear 1 Occasional fine scale, faint erythema, and barely perceptible plaque thickness Mild 2 Fine scale with light coloration and mild plaque elevation Moderate 3 Coarse scale with moderate red coloration and moderate plaque thickness Severe 4 Thick tenacious scale with deep coloration and severe plaque thickness Efficacy evaluation was carried out at Week 8 with treatment success being defined as a score of "clear" (grade 0) or "almost clear" (grade 1) and at least 2 grade improvement from the baseline score. Approximately 30% of enrolled subjects were graded as "mild" on the ISGA scale. The study population ranged in age from 12 to 89 years with 10 subjects less than 18 years of age at baseline. The subjects were 54% male and 88% Caucasian. Table 2 presents the efficacy results for each trial.
Table 2. Number and Percent of Subjects Achieving Success for Body at Week 8 in Each Trial Trial 1 Trial 2 SORILUX Foam
N = 223Vehicle Foam
N = 113SORILUX Foam
N = 214Vehicle Foam
N = 109Number (%) of Subjects with Treatment Success 31 (14%) 8 (7%) 58 (27%) 17 (16%) In one trial, subjects graded as "mild" at baseline showed a greater response to vehicle than SORILUX Foam.
Table 3 presents the success rates by disease severity at baseline for each trial.
Table 3. Number and Percent of Subjects Achieving Success for Body by Baseline ISGA Score and by Trial Trial 1 Trial 2 ISGA Scores at Baseline SORILUX Foam
(N = 223)Vehicle Foam
(N = 113)SORILUX Foam
(N = 214)Vehicle Foam
(N = 109)Mild 2/73 (2.7%) 3/34 (8.8%) 8/56 (14.3%) 4/31 (12.9%) Moderate 29/150 (19.3%) 5/79 (6.3%) 50/158 (31.6%) 13/78 (16.7%) In another multi-center, randomized, double-blind, vehicle-controlled clinical trial, a total of 363 subjects with moderate plaque psoriasis of the scalp and body were randomized 1:1 to SORILUX Foam or vehicle. Subjects applied the assigned treatment to the affected areas twice daily for 8 weeks.
Baseline disease severity of the scalp was graded using a 6-point ISGA; a score of "moderate" corresponded to grade 3.
The primary efficacy evaluation for scalp involvement was carried out at Week 8 with treatment success being defined as a score of "clear" (grade 0) or "almost clear" (grade 1). The study population ranged in age from 12 to 97 years with 11 subjects less than 18 years of age at baseline. The subjects were 60% male and 87% Caucasian. Table 4 presents the efficacy results for the trial.
Table 4. Number and Percent of Subjects Achieving Success for Scalp at Week 8 Trial 3 SORILUX Foam
N = 181Vehicle Foam
N = 182Number (%) of Subjects with Treatment Success 74 (41%) 44 (24%) The contribution to efficacy of individual components of the vehicle has not been established.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
SORILUX (calcipotriene) Foam, 0.005%, is supplied as follows:
60 g aluminum can NDC: 51862-376-60 120 g aluminum can NDC: 51862-376-12 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform the patient to adhere to the following instructions:
- Apply SORILUX Foam to the affected skin areas.
- Apply SORILUX Foam to the scalp when the hair is dry.
- Avoid fire, flame, and smoking during and immediately following application since SORILUX Foam is flammable.
- Avoid contact with the face and eyes. If SORILUX Foam gets on the face or in or near their eyes, rinse thoroughly with water.
- Talk to your doctor if your skin does not improve after treatment with SORILUX Foam for 8 weeks.
- Wash your hands after applying SORILUX Foam unless your hands are the affected site.
- Do not place SORILUX Foam in the refrigerator or freezer.
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
Patient Information
SORILUX (SOR-i-lux)
(calcipotriene)
Foam, 0.005%This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 11/2019 Important: SORILUX Foam is for use on the skin only (topical use). Do not use SORILUX Foam in your mouth, eyes, or vagina. WHAT IS SORILUX FOAM?
SORILUX Foam is a prescription medicine used on the skin (topical) to treat plaque psoriasis of the scalp and body in people 4 years of age and older.
It is not known if SORILUX Foam is safe and effective in people under 4 years old.Do not use SORILUX Foam if you have been told by your healthcare provider that you have a high level of calcium in your blood (hypercalcemia). BEFORE USING SORILUX FOAM, TELL YOUR HEALTHCARE PROVIDER ABOUT ALL YOUR MEDICAL CONDITIONS, INCLUDING IF YOU: - are pregnant or planning to become pregnant. It is not known if SORILUX Foam will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if SORILUX Foam passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with SORILUX Foam.
Know the medicines you take. Keep a list of your medicines with you to show your healthcare provider and pharmacist when you get a new medicine.HOW SHOULD I USE SORILUX FOAM? - Use SORILUX Foam exactly as your healthcare provider tells you to use it. See the detailed "Instructions for Use" at the end of this leaflet for directions about how to apply SORILUX Foam the right away.
- SORILUX Foam is usually applied to the affected skin areas 2 times each day.
- For your scalp, apply SORILUX Foam when your hair is dry.
- Talk with your healthcare provider if your skin does not improve after 8 weeks of treatment with SORILUX Foam.
- Wash your hands after applying SORILUX Foam unless you are using the medicine to treat your hands.
WHAT SHOULD I AVOID WHILE USING SORILUX FOAM? - SORILUX Foam is flammable. Avoid fire, flame, and smoking during and right after you apply SORILUX Foam.
- Avoid getting SORILUX Foam on your face or in or near your eyes. If SORILUX Foam gets on your face or in your eyes, rinse well with water.
WHAT ARE THE POSSIBLE SIDE EFFECTS OF SORILUX FOAM?
SORILUX Foam may cause serious side effects, including:- Too much calcium in your blood. Your healthcare provider may tell you to stop using SORILUX Foam until your calcium levels become normal.
These are not all the possible side effects of SORILUX Foam.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Mayne Pharma at 1-844-825-8500.HOW SHOULD I STORE SORILUX FOAM? - Store SORILUX Foam at room temperature, between 68°F to 77°F (20C° to 25°C).
- Do not store SORILUX Foam in the refrigerator or freezer.
- Do not expose SORILUX Foam to heat or store at temperatures above 120°F (49°C).
- Do not puncture or burn the SORILUX Foam can.
GENERAL INFORMATION ABOUT THE SAFE AND EFFECTIVE USE OF SORILUX FOAM.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use SORILUX Foam for a condition for which it was not prescribed. Do not give SORILUX Foam to other people even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about SORILUX Foam that is written for health professionals.WHAT ARE THE INGREDIENTS IN SORILUX FOAM?
Active ingredient: calcipotriene
Inactive ingredients: cetyl alcohol, dibasic sodium phosphate, dl-α-tocopherol, edetate disodium, isopropyl myristate, light mineral oil, polyoxyl 20 cetostearyl ether, propylene glycol, purified water, stearyl alcohol, and white petrolatum. The foam is dispensed from an aluminum can pressurized with a hydrocarbon (propane/n-butane/isobutane) propellant.
Distributed by:
Mayne Pharma
Greenville, NC 27834
SORILUX is a registered trademark of Mayne Pharma LLC. -
Instructions for UseSORILUX (SOR-i-lux)(calcipotriene)Foam, 0.005%
Read this Instructions for Use before you start using SORILUX Foam and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
Important Information:
- SORILUX Foam is for use on the skin only (topical use). Do not use SORILUX Foam in your mouth, eyes, or vagina.
- Avoid getting SORILUX Foam on your face or in your eyes. If you accidentally get SORILUX Foam on your face or in your eyes, rinse well with water.
How to apply SORILUX Foam to your body:
Follow your healthcare providers instructions on how much SORILUX Foam to use and where to use it. Wash your hands after applying SORILUX Foam unless you are treating areas on your hands.
Step 1. Before applying SORILUX Foam for the first time, break the tiny plastic piece at the base of the can's rim by gently pushing back (away from the piece) on the nozzle. See Figure A.
Step 2: Shake the can of SORILUX Foam before use. See Figure B.
Step 3: Turn the can of SORILUX Foam upside down and press the nozzle. See Figure C.
Step 4. Dispense a small amount of SORILUX Foam into the palm of your hand. See Figure D.
Step 5. Use enough SORILUX Foam to cover the affected area with a thin layer. Gently rub the foam into the affected area until it disappears into the skin. See Figures E and F
How to apply SORILUX Foam to your scalp:
Step 6. Apply SORILUX Foam to your scalp when your hair is dry. Part your hair and apply directly on the affected area. See Figure G. Wash your hands after applying SORILUX Foam.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by: Mayne Pharma, Greenville, NC 27834
Revised: 11/2019
- PRINCIPAL DISPLAY PANEL - 60 gram Can Carton
-
INGREDIENTS AND APPEARANCE
SORILUX
calcipotriene aerosol, foamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51862-376 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength calcipotriene (UNII: 143NQ3779B) (calcipotriene - UNII:143NQ3779B) calcipotriene 50 ug in 1 g Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) EDETATE DISODIUM (UNII: 7FLD91C86K) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LIGHT MINERAL OIL (UNII: N6K5787QVP) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) .ALPHA.-TOCOPHEROL, DL- (UNII: 7QWA1RIO01) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51862-376-60 1 in 1 CARTON 04/11/2017 1 60 g in 1 CAN; Type 0: Not a Combination Product 2 NDC: 51862-376-12 1 in 1 CARTON 04/11/2017 2 120 g in 1 CAN; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022563 04/11/2017 Labeler - Mayne Pharma (867220261) Establishment Name Address ID/FEI Business Operations DPT Laboratories, Ltd. 832224526 ANALYSIS(51862-376) , MANUFACTURE(51862-376) Establishment Name Address ID/FEI Business Operations DPT Laboratories, Ltd. 832224591 PACK(51862-376) , LABEL(51862-376)
Trademark Results [SORILUX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SORILUX 85556472 4552190 Live/Registered |
MAYNE PHARMA, LLC 2012-02-29 |
 SORILUX 85070034 not registered Dead/Abandoned |
Stiefel Laboratories, Inc. 2010-06-23 |
 SORILUX 85064627 not registered Dead/Abandoned |
Stiefel Laboratories, Inc. 2010-06-16 |
 SORILUX 85058503 not registered Dead/Abandoned |
Stiefel Laboratories, Inc. 2010-06-09 |
 SORILUX 77604019 not registered Dead/Abandoned |
Stiefel Laboratories, Inc. 2008-10-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 Figure A
Figure A Figure B
Figure B Figure C
Figure C Figure D
Figure D Figure E
Figure E Figure F
Figure F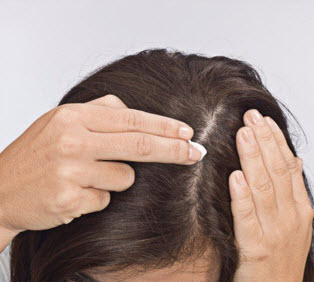 Figure G
Figure G