LACTATED RINGERS- sodium chloride, soldium lactate, potassium chloride and calcium chloride irrigant
Lactated Ringers by
Drug Labeling and Warnings
Lactated Ringers by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Lactated Ringer’s Irrigation is a sterile, nonpyrogenic, isotonic solution in a single dose ARTHROMATIC plastic container for use as an arthroscopic irrigating solution. Each liter contains 6.0 g Sodium Chloride, USP, (NaCl), 3.1 g Sodium Lactate (C3H5NaO3), 300 mg Potassium Chloride, USP, (KCl), and 200 mg Calcium Chloride, USP, (CaCl22H2O). pH 6.5 (6.0 to 7.5). Milliequivalents per liter: Sodium - 130, Potassium - 4, Calcium - 3, Chloride - 109, Lactate - 28. Osmolarity 273 mOsmol/L (calc.). No antimicrobial agent has been added.
The ARTHROMATIC plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexylphthalate (DEHP), up to 5 parts per million. However, the safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by tissue culture toxicity studies.
-
CLINICAL PHARMACOLOGY
Lactated Ringer’s Irrigation is useful as an irrigating fluid for body joints because it approximates the electrolyte composition of synovial fluid, and provides a transparent fluid medium with optical properties suitable for good visualization of the interior joint surface during endoscopic examination. During arthroscopic surgical procedures, the solution acts as a lavage for removing blood, tissue fragments, and bone fragments.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Absorption of large volume of irrigation fluids through a perforation or open wound may result in circulatory overload, cardiac failure, or electrolyte disturbances and acid-base imbalance.
Patients and fluid balance should be monitored accordingly. If absorption of clinically relevant amounts of fluid is suspected, the fluid administration should be interrupted, and the patient evaluated for possible adverse consequences.
Particularly close monitoring is required in patients with:
- Severely impaired renal function
- Impaired cardiac function, or
- Clinical states in which there is edema with sodium retention as a fluid overload syndrome may develop in these patients following absorption of even small quantities of irrigation fluid.
Appropriate therapy should be initiated, as indicated.
Lactated Ringer’s Irrigation must not be used when types of electrocautery are used that are not safe and effective when performed in the presence of electrolyte solutions.
Excessive volume or pressure during irrigation may cause excessive fluid absorption, undue distention of cavities, and/or disruption of tissue.
-
PRECAUTIONS
The container must not be vented.
Vented administration sets with the vent in the open position should not be used with flexible plastic containers. Use of a vented administration set with the vent in the open position could result in air embolism.
Pressurizing solutions contained in flexible plastic containers to increase flow rates can result in air embolism if the residual air in the container is not fully evacuated prior to administration.
Do not connect flexible plastic containers in series in order to avoid air embolism due to possible residual air contained in the primary container.
Drug Interactions
If clinically relevant amounts of solution have been absorbed, potential interactions with other agents must be considered, such as:
Patients treated with drugs that may increase the risk of sodium and fluid retention, such as corticosteroids and carbenoxolone, may have an increased risk of sodium and fluid retention.
Due to the alkalinizing action of lactate (formation of bicarbonate), Lactated Ringer’s Irrigation may interfere with the elimination of drugs for which renal elimination is pH dependent:
- Renal clearance of acidic drugs such as salicylates, barbiturates, and lithium may be increased.
- Renal clearance of alkaline drugs, such as sympathomimetics (e.g., ephedrine, pseudoephedrine), dextroamphetamine (dexamphetamine) sulfate, and fenfluramine (phenfluramine) hydrochloride may be decreased.
The risk of hyperkalemia is increased in patients treated with agents or products that can cause hyperkalemia or increase the risk of hyperkalemia, such as potassium-sparing diuretics (amiloride, spironolactone, triameterene), with ACE inhibitors, angiotensin II receptor antagonists, or the immunosuppressants tacrolimus and cyclosporine.
Administration of potassium in patients treated with such medications can produce severe and potentially fatal hyperkalemia, particularly in patients with severe renal insufficiency.
Absorption of calcium-containing solutions may increase the effects of digitalis and lead to serious or fatal cardiac arrhythmia.
In patients treated with thiazide diuretics or vitamin D, the risk of hypercalcemia is increased.
Pregnancy
Teratogenic Effects
Animal reproduction studies have not been conducted with Lactated Ringer’s Irrigation. It is also not known whether Lactated Ringer’s Irrigation can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Lactated Ringer’s Irrigation should be given to a pregnant woman only if clearly needed.
Nursing Mothers
There is no adequate data from the use of Lactated Ringer’s Irrigation in lactating women. Physicians should carefully consider the potential risks and benefits for each specific patient before prescribing Lactated Ringer’s Irrigation.
-
ADVERSE REACTIONS
Post-Marketing Adverse Reactions
No adverse reactions were identified in Baxter’s Adverse Event Reporting System database with Lactated Ringer’s Irrigation.
Class Reactions
Adverse reactions reported with Lactated Ringer’s Irrigation (manufacturer unspecified) are: Fluid absorption manifested by Pulmonary edema, Edema, and Electrolyte disturbances.
Though not indicated for intravenous administration, absorption of irrigation fluid into tissue or vasculature through a perforation or open wound is possible. As a result, adverse reactions reported with Lactated Ringer’s solutions with and without Dextrose for intravenous administration may be applicable and include:
- Hypersensitivity reactions, including Anaphylactic/Anaphylactoid reactions, with the following manifestations: Angioedema, Chest pain, Chest discomfort, Decreased heart rate, Tachycardia, Blood pressure decreased, Respiratory distress, Bronchospasm, Dyspnea, Cough, Urticaria, Rash, Pruritus, Erythema, Flushing, Throat irritation, Paresthesias, Hypoesthesia oral, Dysgeusia, Nausea, Anxiety, Headache, Hyperkalemia
- Pyrexia
-
DOSAGE AND ADMINISTRATION
The volume and/or rate of solution needed will vary with the nature and duration of the arthroscopic procedure.
This solution, as it is packaged, is not intended for IV administration or injection.
The container must not be vented (See Precautions).
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Do not administer unless the solution is clear and the seal is intact. If particulate matter or discoloration is found, contact Baxter Customer Service
Aseptic technique should be used when applying this product.
The contents of an opened container should be used promptly to minimize the possibility of bacterial growth or pyrogen formation. Discard the unused portion of irrigating solution since no antimicrobial agent has been added.
When using Lactated Ringer’s Irrigation for pour irrigation, prevent contact of the fluid with the external surface of the container.
Lactated Ringer’s Irrigation is for single-patient use only.
Microwave heating of irrigation fluids is not recommended. If desired, Lactated Ringer’s Irrigation may be warmed in a water bath or oven to not more than 45° C while maintaining sterility. Lactated Ringer’s Irrigation that has been warmed must not be returned to storage.
Cetriaxone must not be mixed with calcium-containing solutions, including Lactated Ringer’s Irrigation.
Additives may be incompatible with Lactated Ringer’s Irrigation. Additives known or determined to be incompatible should not be used.
As with all parenteral solutions, compatibility of the additives with the solution must be assessed before addition, by checking for, for example, a possible color change and/or the appearance of precipitates, insoluble complexes, or crystals. Before adding a substance or medication, verify that it is soluble and/or stable in water and that the pH range of Lactated Ringer’s Irrigation is appropriate.
The instructions for use of the medication to be added and other relevant literature must be consulted.
When making additions to Lactated Ringer’s Irrigation, aseptic technique must be used. Mix the solution thoroughly when additives have been introduced. Do not store solutions containing additives.
-
HOW SUPPLIED
Lactated Ringer’s Irrigation in ARTHROMATIC Plastic Container is available as follows:
2B7487
3000 mL
NDC: 0338-0137-27
2B7489
5000 mL
NDC: 0338-0137-29
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored at room temperature (25° C); brief exposure up to 40° C does not adversely affect the product.
-
DIRECTIONS FOR USE
Tear overwrap down side at slit and remove solution container. Visually inspect the container. If the outlet port protector is damaged, detached, or not present, discard container as solution path sterility may be impaired. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for minute leaks by squeezing bag firmly. If leaks are found, discard solution as sterility may be impaired.
Use Aseptic Technique.
- 1. Suspend container using hanger hole.
- 2. Remove protector from outlet port.
- 3. Attach irrigation set. Refer to complete directions accompanying set.
- SPL UNCLASSIFIED SECTION
-
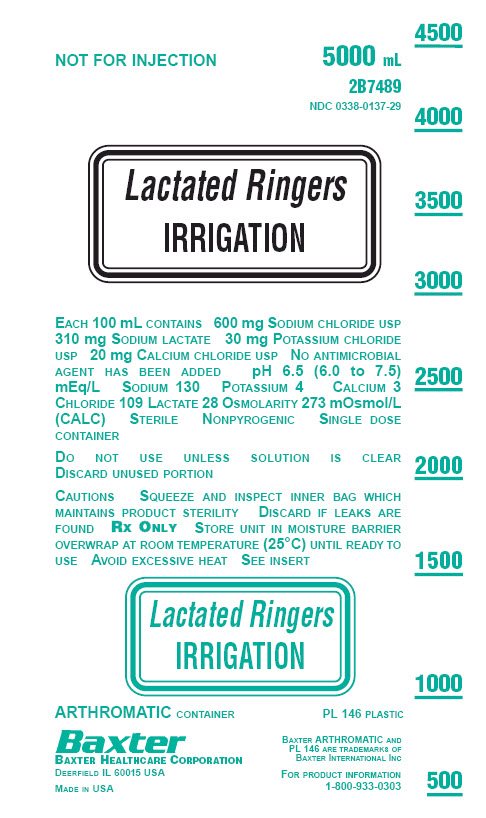
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
NOT FOR INJECTION
5000 mL
2B7489
NDC: 0338-0137-29
Lactated Ringers
IRRIGATION
EACH 100 mL CONTAINS 600 mg SODIUM CHLORIDE USP
310 mg SODIUM LACTATE 30 mg POTASSIUM CHLORIDE
USP 20 mg CALCIUM CHLORIDE USP NO ANTIMICROBIAL
AGENT HAS BEEN ADDED pH 6.5 (6.0 to 7.5)
mEq/L SODIUM 130 POTASSIUM 4 CALCIUM 3
CHLORIDE 109 LACTATE 28 OSMOLARITY 273 mOsmol/L
(CALC) STERILE NONPYROGENIC SINGLE DOSE
CONTAINERDO NOT USE UNLESS SOLUTION IS CLEAR
DISCARD UNUSED PORTIONCAUTIONS SQUEEZE AND INSPECT INNER BAG WHICH
MAINTAINS PRODUCT STERILITY DISCARD IF LEAKS ARE
FOUND RX ONLY STORE UNIT IN MOISTURE BARRIER
OVERWRAP AT ROOM TEMPERATURE (25°C) UNTIL READY TO
USE AVOID EXCESSIVE HEAT SEE INSERTLactated Ringers
IRRIGATION
ARTHROMATIC CONTAINER
PL 146 PLASTIC
Baxter
BAXTER HEALTHCARE CORPORATION
DEERFIELD IL 60015 USAMADE IN USA
BAXTER ARTHROMATIC AND
PL 146 ARE TRADEMARKS OF
BAXTER INTERNATIONAL INCFOR PRODUCT INFORMATION
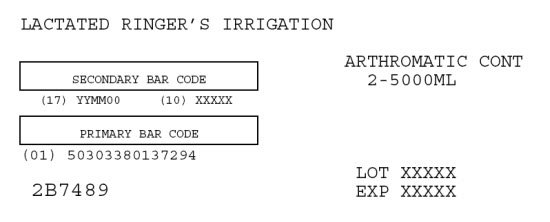
1-800-933-0303LACTATED RINGER’S IRRIGATION
ARTHROMATIC CONT
2-5000MLSECONDARY BAR CODE
(17) YYMM00 (10) XXXXXPRIMARY BAR CODE
(01) 50303380137294LOT XXXXX
EXP XXXXX2B7489
-
INGREDIENTS AND APPEARANCE
LACTATED RINGERS
sodium chloride, soldium lactate, potassium chloride and calcium chloride irrigantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-0137 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 6 g in 1000 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID - UNII:33X04XA5AT) SODIUM LACTATE 3.1 g in 1000 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152, CHLORIDE ION - UNII:Q32ZN48698) POTASSIUM CHLORIDE 300 mg in 1000 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 200 mg in 1000 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0137-29 5000 mL in 1 BAG; Type 0: Not a Combination Product 04/03/1984 2 NDC: 0338-0137-27 3000 mL in 1 BAG; Type 0: Not a Combination Product 04/03/1984 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018921 04/03/1984 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 059140764 ANALYSIS(0338-0137) , MANUFACTURE(0338-0137) , LABEL(0338-0137) , PACK(0338-0137) , STERILIZE(0338-0137) , API MANUFACTURE(0338-0137) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 194684502 ANALYSIS(0338-0137)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.