GENTLE REJUVENATION ULTRA LIGHT REPAIR SPF 30 SUNSCREEN- homosalate, octinoxate, octocrylene, and zinc oxide cream
GENTLE REJUVENATION ULTRA LIGHT REPAIR SPF 30 by
Drug Labeling and Warnings
GENTLE REJUVENATION ULTRA LIGHT REPAIR SPF 30 by is a Otc medication manufactured, distributed, or labeled by Obagi Medical Products, Inc., a division of Valeant Pharmaceuticals North America LLC., LABORATOIRE DR RENAUD INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
-
Inactive ingredients
aluminum starch octenylsuccinate, behentrimonium methosulfate, boron nitride, carbomer, ceramide AP, ceramide EOP, ceramide NP, cetearyl alcohol, cholesterol, dimethicone, disodium EDTA, ethoxydiglycol, glycerin, hydroxyethylcellulose, kinetin, methylparaben, niacinamide, PEG-12 glyceryl dimyristate, phytosphingosine, propylparaben, sodium hyaluronate, sodium lauroyl lactylate, water, xanthan gum, zeatin
- Other information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
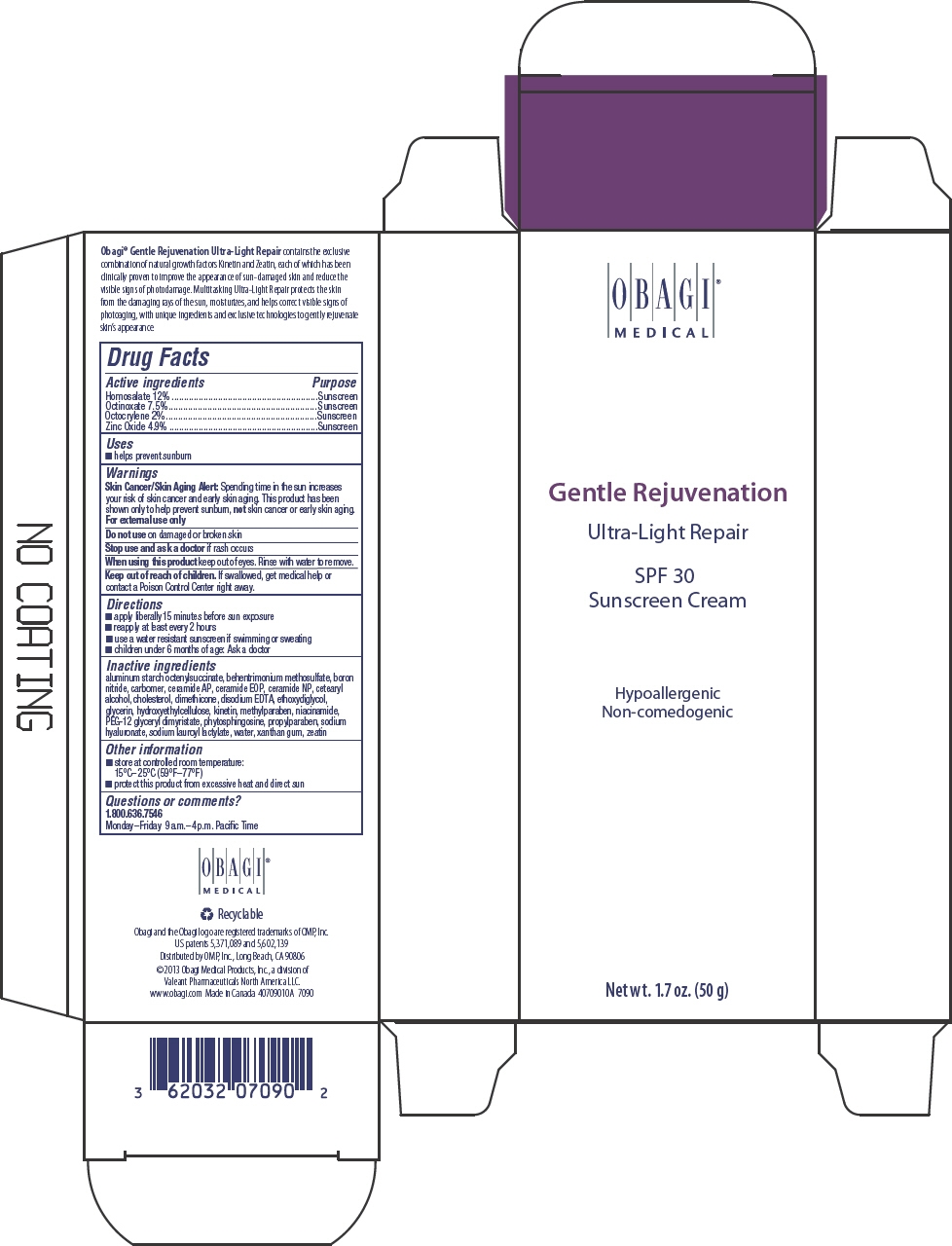
- PRINCIPAL DISPLAY PANEL - 50 g Tube Carton
-
INGREDIENTS AND APPEARANCE
GENTLE REJUVENATION ULTRA LIGHT REPAIR SPF 30 SUNSCREEN
homosalate, octinoxate, octocrylene, and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62032-131 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 120 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 20 mg in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 49 mg in 1 g Inactive Ingredients Ingredient Name Strength ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) BEHENTRIMONIUM METHOSULFATE (UNII: 5SHP745C61) BORON NITRIDE (UNII: 2U4T60A6YD) CARBOMER HOMOPOLYMER TYPE B (ALLYL SUCROSE CROSSLINKED) (UNII: Z135WT9208) CERAMIDE 6 II (UNII: F1X8L2B00J) CERAMIDE 1 (UNII: 5THT33P7X7) CERAMIDE 3 (UNII: 4370DF050B) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CHOLESTEROL (UNII: 97C5T2UQ7J) DIMETHICONE 100 (UNII: RO266O364U) EDETATE DISODIUM (UNII: 7FLD91C86K) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) GLYCERIN (UNII: PDC6A3C0OX) KINETIN (UNII: P39Y9652YJ) METHYLPARABEN (UNII: A2I8C7HI9T) NIACINAMIDE (UNII: 25X51I8RD4) PEG-12 GLYCERYL DIMYRISTATE (UNII: VS4W16AQ3X) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) PROPYLPARABEN (UNII: Z8IX2SC1OH) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) ZEATIN (UNII: 7I6OOJ9GR6) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62032-131-70 1 in 1 CARTON 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 10/15/2013 Labeler - Obagi Medical Products, Inc., a division of Valeant Pharmaceuticals North America LLC. (790553353) Establishment Name Address ID/FEI Business Operations LABORATOIRE DR RENAUD INC. 202501565 MANUFACTURE(62032-131) , LABEL(62032-131) , PACK(62032-131)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.