VARIVAX- varicella virus vaccine live injection, powder, lyophilized, for suspension
VARIVAX by
Drug Labeling and Warnings
VARIVAX by is a Other medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VARIVAX safely and effectively. See full prescribing information for VARIVAX.
VARIVAX®
Varicella Virus Vaccine Live
Suspension for subcutaneous injection
Initial U.S. Approval: 1995RECENT MAJOR CHANGES
Warnings and Precautions Risk of Vaccine Virus Transmission (5.4) 03/2020 INDICATIONS AND USAGE
VARIVAX is a vaccine indicated for active immunization for the prevention of varicella in individuals 12 months of age and older. (1)
DOSAGE AND ADMINISTRATION
Each dose is approximately 0.5 mL after reconstitution and is administered by subcutaneous injection. (2.1)
Children (12 months to 12 years of age)
- If a second dose is administered, there should be a minimum interval of 3 months between doses. (2.1)
Adolescents (≥13 years of age) and Adults
- Two doses, to be administered a minimum of 4 weeks apart. (2.1)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- History of severe allergic reaction to any component of the vaccine (including neomycin and gelatin) or to a previous dose of varicella vaccine. (4.1)
- Primary or acquired immunodeficiency states. (4.2)
- Any febrile illness or active infection, including untreated tuberculosis. (4.3)
- Pregnancy. (4.4, 8.1, 17)
WARNINGS AND PRECAUTIONS
- Evaluate individuals for immune competence prior to administration of VARIVAX if there is a family history of congenital or hereditary immunodeficiency. (5.2)
- Avoid close contact with high-risk individuals susceptible to varicella because of possible transmission of varicella vaccine virus. (5.4)
- Defer vaccination for at least 5 months following blood or plasma transfusions, or administration of immune globulins (IG). (5.5, 7.2)
- Avoid use of salicylates for 6 weeks following administration of VARIVAX to children and adolescents. (5.6, 7.1)
ADVERSE REACTIONS
- Frequently reported (≥10%) adverse reactions in children ages 1 to 12 years include:
- fever ≥102.0°F (38.9°C) oral: 14.7%
- injection-site complaints: 19.3% (6.1)
- Frequently reported (≥10%) adverse reactions in adolescents and adults ages 13 years and older include:
- fever ≥100.0°F (37.8°C) oral: 10.2%
- injection-site complaints: 24.4% (6.1)
- Other reported adverse reactions in all age groups include:
- varicella-like rash (injection site)
- varicella-like rash (generalized) (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
DRUG INTERACTIONS
- Reye syndrome has been reported in children and adolescents following the use of salicylates during wild-type varicella infection. (5.6, 7.1)
- Passively acquired antibodies from blood, plasma, or immunoglobulin potentially may inhibit the response to varicella vaccination. (5.5, 7.2)
- Tuberculin skin testing may be performed before VARIVAX is administered or on the same day, or six weeks following vaccination with VARIVAX. (7.3)
USE IN SPECIFIC POPULATIONS
Pregnancy: Do not administer VARIVAX to females who are pregnant. Pregnancy should be avoided for 3 months following vaccination with VARIVAX. (4.4, 8.1, 17)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose and Schedule
2.2 Reconstitution Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Severe Allergic Reaction
4.2 Immunosuppression
4.3 Concurrent Illness
4.4 Pregnancy
5 WARNINGS AND PRECAUTIONS
5.1 Management of Allergic Reactions
5.2 Family History of Immunodeficiency
5.3 Use in HIV-Infected Individuals
5.4 Risk of Vaccine Virus Transmission
5.5 Immune Globulins and Transfusions
5.6 Salicylate Therapy
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Salicylates
7.2 Immune Globulins and Transfusions
7.3 Tuberculin Skin Testing
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.6 Duration of Protection
14 CLINICAL STUDIES
14.1 Clinical Efficacy
14.2 Immunogenicity
14.3 Persistence of Immune Response
14.4 Studies with Other Vaccines
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Subcutaneous administration only
2.1 Recommended Dose and Schedule
VARIVAX is administered as an approximately 0.5-mL dose by subcutaneous injection into the outer aspect of the upper arm (deltoid region) or the anterolateral thigh.
Do not administer this product intravascularly or intramuscularly.
Children (12 months to 12 years of age)
If a second dose is administered, there should be a minimum interval of 3 months between doses [see Clinical Studies (14.1)].
Adolescents (≥13 years of age) and Adults
Two doses of vaccine, to be administered with a minimum interval of 4 weeks between doses [see Clinical Studies (14.1)].
2.2 Reconstitution Instructions
When reconstituting the vaccine, use only the sterile diluent supplied with VARIVAX. The sterile diluent does not contain preservatives or other anti-viral substances which might inactivate the vaccine virus.
Use a sterile syringe free of preservatives, antiseptics, and detergents for each reconstitution and injection of VARIVAX because these substances may inactivate the vaccine virus.
To reconstitute the vaccine, first withdraw the total volume of provided sterile diluent into a syringe. Inject all of the withdrawn diluent into the vial of lyophilized vaccine and gently agitate to mix thoroughly. Withdraw the entire contents into the syringe, inject the total volume (approximately 0.5 mL) of reconstituted vaccine subcutaneously, and discard vial. VARIVAX, when reconstituted, is a clear, colorless to pale yellow liquid.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use the product if particulates are present or if it appears discolored.
To minimize loss of potency, administer VARIVAX immediately after reconstitution. Discard if reconstituted vaccine is not used within 30 minutes.
Do not freeze reconstituted vaccine.
Do not combine VARIVAX with any other vaccine through reconstitution or mixing.
-
3 DOSAGE FORMS AND STRENGTHS
VARIVAX is a suspension for injection supplied as a single-dose vial of lyophilized vaccine to be reconstituted using the accompanying sterile diluent [see Dosage and Administration (2.2) and How Supplied/Storage and Handling (16)]. A single dose after reconstitution is approximately 0.5 mL.
-
4 CONTRAINDICATIONS
4.1 Severe Allergic Reaction
Do not administer VARIVAX to individuals with a history of anaphylactic or severe allergic reaction to any component of the vaccine (including neomycin and gelatin) or to a previous dose of a varicella-containing vaccine.
4.2 Immunosuppression
Do not administer VARIVAX to immunosuppressed or immunodeficient individuals, including those with a history of primary or acquired immunodeficiency states, leukemia, lymphoma or other malignant neoplasms affecting the bone marrow or lymphatic system, AIDS, or other clinical manifestations of infection with human immunodeficiency virus (HIV).
Do not administer VARIVAX to individuals receiving immunosuppressive therapy, including individuals receiving immunosuppressive doses of corticosteroids.
VARIVAX is a live, attenuated varicella-zoster vaccine (VZV) and may cause an extensive vaccine-associated rash or disseminated disease in individuals who are immunosuppressed or immunodeficient.
4.3 Concurrent Illness
Do not administer VARIVAX to individuals with any febrile illness. Do not administer VARIVAX to individuals with active, untreated tuberculosis.
4.4 Pregnancy
Do not administer VARIVAX to individuals who are pregnant because the effects of the vaccine on fetal development are unknown. Wild-type varicella (natural infection) is known to sometimes cause fetal harm. If vaccination of postpubertal females is undertaken, pregnancy should be avoided for three months following vaccination [see Use in Specific Populations (8.1) and Patient Counseling Information (17)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Management of Allergic Reactions
Adequate treatment provisions, including epinephrine injection (1:1000), should be available for immediate use should anaphylaxis occur.
5.2 Family History of Immunodeficiency
Vaccination should be deferred in patients with a family history of congenital or hereditary immunodeficiency until the patient's immune status has been evaluated and the patient has been found to be immunocompetent.
5.3 Use in HIV-Infected Individuals
The Advisory Committee for Immunization Practices (ACIP) has recommendations on the use of varicella vaccine in HIV-infected individuals.
5.4 Risk of Vaccine Virus Transmission
Post-marketing experience suggests that transmission of varicella vaccine virus (Oka/Merck) resulting in varicella infection including disseminated disease may occur rarely between vaccine recipients (who develop or do not develop a varicella-like rash) and contacts susceptible to varicella including healthy as well as high-risk individuals.
Due to the concern for transmission of vaccine virus, vaccine recipients should attempt to avoid whenever possible close association with susceptible high-risk individuals for up to six weeks following vaccination with VARIVAX. Susceptible high-risk individuals include:
- Immunocompromised individuals;
- Pregnant women without documented history of varicella or laboratory evidence of prior infection;
- Newborn infants of mothers without documented history of varicella or laboratory evidence of prior infection and all newborn infants born at <28 weeks gestation regardless of maternal varicella immunity.
5.5 Immune Globulins and Transfusions
Immunoglobulins should not be given concomitantly with VARIVAX. Vaccination should be deferred for at least 5 months following blood or plasma transfusions, or administration of immune globulin(s) {1}.
Following administration of VARIVAX, immune globulin(s) should not be given for 2 months thereafter unless its use outweighs the benefits of vaccination {1}. [See Drug Interactions (7.2).]
5.6 Salicylate Therapy
Avoid use of salicylates (aspirin) or salicylate-containing products in children and adolescents 12 months through 17 years of age for six weeks following vaccination with VARIVAX because of the association of Reye syndrome with aspirin therapy and wild-type varicella infection. [See Drug Interactions (7.1).]
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in clinical practice. Vaccine-related adverse reactions reported during clinical trials were assessed by the study investigators to be possibly, probably, or definitely vaccine-related and are summarized below.
In clinical trials {2-9}, VARIVAX was administered to over 11,000 healthy children, adolescents, and adults.
In a double-blind, placebo-controlled study among 914 healthy children and adolescents who were serologically confirmed to be susceptible to varicella, the only adverse reactions that occurred at a significantly (p<0.05) greater rate in vaccine recipients than in placebo recipients were pain and redness at the injection site {2}.
Children 1 to 12 Years of Age
One-Dose Regimen in Children
In clinical trials involving healthy children monitored for up to 42 days after a single dose of VARIVAX, the frequency of fever, injection-site complaints, or rashes were reported as shown in Table 1:
Table 1: Fever, Local Reactions, and Rashes (%) in Children 1 to 12 Years of Age 0 to 42 Days After Receipt of a Single Dose of VARIVAX Reaction N % Experiencing Reaction Peak Occurrence During Postvaccination Days Fever ≥102.0°F (38.9°C) Oral
8827 14.7% 0 to 42 Injection-site complaints 8916 19.3% 0 to 2 (pain/soreness, swelling and/or erythema, rash, pruritus, hematoma, induration, stiffness) Varicella-like rash (injection site)
8916 3.4% 8 to 19 Median number of lesions 2 Varicella-like rash (generalized)
8916 3.8% 5 to 26 Median number of lesions 5 In addition, adverse events occurring at a rate of ≥1% are listed in decreasing order of frequency: upper respiratory illness, cough, irritability/nervousness, fatigue, disturbed sleep, diarrhea, loss of appetite, vomiting, otitis, diaper rash/contact rash, headache, teething, malaise, abdominal pain, other rash, nausea, eye complaints, chills, lymphadenopathy, myalgia, lower respiratory illness, allergic reactions (including allergic rash, hives), stiff neck, heat rash/prickly heat, arthralgia, eczema/dry skin/dermatitis, constipation, itching.
Pneumonitis has been reported rarely (<1%) in children vaccinated with VARIVAX.
Febrile seizures have occurred at a rate of <0.1% in children vaccinated with VARIVAX.
Two-Dose Regimen in Children
Nine hundred eighty-one (981) subjects in a clinical trial received 2 doses of VARIVAX 3 months apart and were actively followed for 42 days after each dose. The 2-dose regimen of varicella vaccine had a safety profile comparable to that of the 1-dose regimen. The overall incidence of injection-site clinical complaints (primarily erythema and swelling) observed in the first 4 days following vaccination was 25.4% Postdose 2 and 21.7% Postdose 1, whereas the overall incidence of systemic clinical complaints in the 42-day follow-up period was lower Postdose 2 (66.3%) than Postdose 1 (85.8%).
Adolescents (13 Years of Age and Older) and Adults
In clinical trials involving healthy adolescents and adults, the majority of whom received two doses of VARIVAX and were monitored for up to 42 days after any dose, the frequencies of fever, injection-site complaints, or rashes are shown in Table 2.
Table 2: Fever, Local Reactions, and Rashes (%) in Adolescents and Adults 0 to 42 Days After Receipt of VARIVAX Reaction N % Post
Dose 1Peak Occurrence in
Postvaccination DaysN % Post
Dose 2Peak Occurrence in
Postvaccination DaysFever ≥100.0°F (37.8°C) Oral
1584 10.2% 14 to 27 956 9.5% 0 to 42 Injection-site complaints 1606 24.4% 0 to 2 955 32.5% 0 to 2 (soreness, erythema, swelling, rash, pruritus, pyrexia, hematoma, induration, numbness) Varicella-like rash (injection site)
1606 3% 6 to 20 955 1% 0 to 6 Median number of lesions 2 2 Varicella-like rash (generalized)
1606 5.5% 7 to 21 955 0.9% 0 to 23 Median number of lesions 5 5.5 In addition, adverse events reported at a rate of ≥1% are listed in decreasing order of frequency: upper respiratory illness, headache, fatigue, cough, myalgia, disturbed sleep, nausea, malaise, diarrhea, stiff neck, irritability/nervousness, lymphadenopathy, chills, eye complaints, abdominal pain, loss of appetite, arthralgia, otitis, itching, vomiting, other rashes, constipation, lower respiratory illness, allergic reactions (including allergic rash, hives), contact rash, cold/canker sore.
6.2 Post-Marketing Experience
Broad use of VARIVAX could reveal adverse events not observed in clinical trials.
The following additional adverse events, regardless of causality, have been reported during post-marketing use of VARIVAX:
Body as a Whole
Anaphylaxis (including anaphylactic shock) and related phenomena such as angioneurotic edema, facial edema, and peripheral edema.
Eye Disorders
Necrotizing retinitis (in immunocompromised individuals).
Hemic and Lymphatic System
Aplastic anemia; thrombocytopenia (including idiopathic thrombocytopenic purpura (ITP)).
Infections and Infestations
Varicella (vaccine strain).
Nervous/Psychiatric
Encephalitis; cerebrovascular accident; transverse myelitis; Guillain-Barré syndrome; Bell's palsy; ataxia; non-febrile seizures; aseptic meningitis; meningitis; dizziness; paresthesia.
Cases of encephalitis or meningitis caused by vaccine strain varicella virus have been reported in immunocompetent individuals previously vaccinated with VARIVAX months to years after vaccination. Reported cases were commonly associated with preceding or concurrent herpes zoster rash. [See Clinical Pharmacology (12.2)].
Respiratory
Pharyngitis; pneumonia/pneumonitis.
Skin
Stevens-Johnson syndrome; erythema multiforme; Henoch-Schönlein purpura; secondary bacterial infections of skin and soft tissue, including impetigo and cellulitis; herpes zoster.
-
7 DRUG INTERACTIONS
7.1 Salicylates
No cases of Reye syndrome have been observed following vaccination with VARIVAX. Vaccine recipients should avoid use of salicylates for 6 weeks after vaccination with VARIVAX, as Reye syndrome has been reported following the use of salicylates during wild-type varicella infection [see Warnings and Precautions (5.6)].
7.2 Immune Globulins and Transfusions
Blood, plasma, and immune globulins contain antibodies that may interfere with vaccine virus replication and decrease the immune response to VARIVAX. Vaccination should be deferred for at least 5 months following blood or plasma transfusions, or administration of immune globulin(s) {1}.
Following administration of VARIVAX, immune globulin(s) should not be given for 2 months thereafter unless its use outweighs the benefits of vaccination {1}. [See Warnings and Precautions (5.5).]
7.3 Tuberculin Skin Testing
Tuberculin skin testing, with tuberculin purified protein derivative (PPD), may be performed before VARIVAX is administered or on the same day, or at least 4 weeks following vaccination with VARIVAX, as other live virus vaccines may cause a temporary depression of tuberculin skin test sensitivity leading to false negative results.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
VARIVAX is contraindicated for use in pregnant women because the vaccine contains live, attenuated varicella virus, and it is known that wild-type varicella virus, if acquired during pregnancy, can cause congenital varicella syndrome [see Contraindications (4.4) and Patient Counseling Information (17)]. No increased risk for miscarriage, major birth defect or congenital varicella syndrome was observed in a pregnancy exposure registry that monitored outcomes after inadvertent use. There are no relevant animal data.
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4%, and 15% to 20%, respectively.
Human Data
A pregnancy exposure registry was maintained from 1995 to 2013 to monitor pregnancy and fetal outcomes following inadvertent administration of VARIVAX. The registry prospectively enrolled 1522 women who received a dose of VARIVAX during pregnancy or within three months prior to conception. After excluding elective terminations (n=60), ectopic pregnancies (n=1) and those lost to follow-up (n=556), there were 905 pregnancies with known outcomes. Of these 905 pregnancies, 271 (30%) were in women who were vaccinated within the three months prior to conception. Miscarriage was reported for 10% of pregnancies (95/905), and major birth defects were reported for 2.6% of live born infants (21/819). These rates of assessed outcomes were consistent with estimated background rates. None of the women who received VARIVAX vaccine delivered infants with abnormalities consistent with congenital varicella syndrome.
8.2 Lactation
Risk Summary
It is not known whether varicella vaccine virus is excreted in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for VARIVAX, and any potential adverse effects on the breastfed child from VARIVAX or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
-
11 DESCRIPTION
VARIVAX [Varicella Virus Vaccine Live] is a preparation of the Oka/Merck strain of live, attenuated varicella virus. The virus was initially obtained from a child with wild-type varicella, then introduced into human embryonic lung cell cultures, adapted to and propagated in embryonic guinea pig cell cultures and finally propagated in human diploid cell cultures (WI-38). Further passage of the virus for varicella vaccine was performed at Merck Research Laboratories (MRL) in human diploid cell cultures (MRC-5) that were free of adventitious agents. This live, attenuated varicella vaccine is a lyophilized preparation containing sucrose, phosphate, glutamate, and processed gelatin as stabilizers.
VARIVAX, when reconstituted as directed, is a sterile preparation for subcutaneous injection. Each approximately 0.5-mL dose contains a minimum of 1350 plaque-forming units (PFU) of Oka/Merck varicella virus when reconstituted and stored at room temperature for a maximum of 30 minutes. Each 0.5-mL dose also contains approximately 25 mg of sucrose, 12.5 mg hydrolyzed gelatin, 3.2 mg of sodium chloride, 0.5 mg of monosodium L-glutamate, 0.45 mg of sodium phosphate dibasic, 0.08 mg of potassium phosphate monobasic, and 0.08 mg of potassium chloride. The product also contains residual components of MRC-5 cells including DNA and protein and trace quantities of sodium phosphate monobasic, EDTA, neomycin and fetal bovine serum. The product contains no preservative.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
VARIVAX induces both cell-mediated and humoral immune responses to varicella-zoster virus. The relative contributions of humoral immunity and cell-mediated immunity to protection from varicella are unknown.
12.2 Pharmacodynamics
Transmission
In the placebo-controlled efficacy trial, transmission of vaccine virus was assessed in household settings (during the 8-week postvaccination period) in 416 susceptible placebo recipients who were household contacts of 445 vaccine recipients. Of the 416 placebo recipients, three developed varicella and seroconverted, nine reported a varicella-like rash and did not seroconvert, and six had no rash but seroconverted. If vaccine virus transmission occurred, it did so at a very low rate and possibly without recognizable clinical disease in contacts. These cases may represent either wild-type varicella from community contacts or a low incidence of transmission of vaccine virus from vaccinated contacts. Post-marketing experience suggests that transmission of varicella vaccine virus (Oka/Merck) resulting in varicella infection including disseminated disease may occur rarely between vaccine recipients (who develop or do not develop a varicella-like rash) and contacts susceptible to varicella including healthy as well as high risk individuals [see Warnings and Precautions (5.4)] {2,12}.
Herpes Zoster
Overall, 9454 healthy children (12 months to 12 years of age) and 1648 adolescents and adults (13 years of age and older) have been vaccinated with VARIVAX in clinical trials. Eight cases of herpes zoster have been reported in children during 42,556 person-years of follow-up in clinical trials, resulting in a calculated incidence of at least 18.8 cases per 100,000 person-years. The completeness of this reporting has not been determined. One case of herpes zoster has been reported in the adolescent and adult age group during 5410 person-years of follow-up in clinical trials, resulting in a calculated incidence of 18.5 cases per 100,000 person-years. All 9 cases were mild and without sequelae. Two cultures (one child and one adult) obtained from vesicles were positive for wild-type VZV as confirmed by restriction endonuclease analysis {13}. The long-term effect of VARIVAX on the incidence of herpes zoster, particularly in those vaccinees exposed to wild-type varicella, is unknown at present.
In children, the reported rate of herpes zoster in vaccine recipients appears not to exceed that previously determined in a population-based study of healthy children who had experienced wild-type varicella {14}. The incidence of herpes zoster in adults who have had wild-type varicella infection is higher than that in children.
The vaccine virus (Oka/Merck strain) contained in VARIVAX may establish latency of varicella zoster virus in immunocompetent individuals, with the potential for later development of herpes zoster [see Adverse Reactions (6.2)].
12.6 Duration of Protection
The duration of protection of VARIVAX is unknown; however, long-term efficacy studies have demonstrated continued protection up to 10 years after vaccination {15} [see Clinical Studies (14.1)]. A boost in antibody levels has been observed in vaccinees following exposure to wild-type varicella which could account for the apparent long-term protection after vaccination in these studies.
-
14 CLINICAL STUDIES
14.1 Clinical Efficacy
The protective efficacy of VARIVAX was established by: (1) a placebo-controlled, double-blind clinical trial, (2) comparing varicella rates in vaccinees versus historical controls, and (3) assessing protection from disease following household exposure.
Clinical Data in Children
One-Dose Regimen in Children
Although no placebo-controlled trial was carried out with VARIVAX using the current vaccine, a placebo-controlled trial was conducted using a formulation containing 17,000 PFU per dose {2,16}. In this trial, a single dose of VARIVAX protected 96 to 100% of children against varicella over a two-year period. The study enrolled healthy individuals 1 to 14 years of age (n=491 vaccine, n=465 placebo). In the first year, 8.5% of placebo recipients contracted varicella, while no vaccine recipient did, for a calculated protection rate of 100% during the first varicella season. In the second year, when only a subset of individuals agreed to remain in the blinded study (n=163 vaccine, n=161 placebo), 96% protective efficacy was calculated for the vaccine group as compared to placebo.
In early clinical trials, a total of 4240 children 1 to 12 years of age received 1000 to 1625 PFU of attenuated virus per dose of VARIVAX and have been followed for up to nine years post single-dose vaccination. In this group there was considerable variation in varicella rates among studies and study sites, and much of the reported data were acquired by passive follow-up. It was observed that 0.3 to 3.8% of vaccinees per year reported varicella (called breakthrough cases). This represents an approximate 83% (95% confidence interval [CI], 82%, 84%) decrease from the age-adjusted expected incidence rates in susceptible subjects over this same period {14}. In those who developed breakthrough varicella postvaccination, the majority experienced mild disease (median of the maximum number of lesions <50). In one study, a total of 47% (27/58) of breakthrough cases had <50 lesions compared with 8% (7/92) in unvaccinated individuals, and 7% (4/58) of breakthrough cases had >300 lesions compared with 50% (46/92) in unvaccinated individuals {17}.
Among a subset of vaccinees who were actively followed in these early trials for up to nine years postvaccination, 179 individuals had household exposure to varicella. There were no reports of breakthrough varicella in 84% (150/179) of exposed children, while 16% (29/179) reported a mild form of varicella (38% [11/29] of the cases with a maximum total number of <50 lesions; no individuals with >300 lesions). This represents an 81% reduction in the expected number of varicella cases utilizing the historical attack rate of 87% following household exposure to varicella in unvaccinated individuals in the calculation of efficacy.
In later clinical trials, a total of 1114 children 1 to 12 years of age received 2900 to 9000 PFU of attenuated virus per dose of VARIVAX and have been actively followed for up to 10 years post single-dose vaccination. It was observed that 0.2% to 2.3% of vaccinees per year reported breakthrough varicella for up to 10 years post single-dose vaccination. This represents an estimated efficacy of 94% (95% CI, 93%, 96%), compared with the age-adjusted expected incidence rates in susceptible subjects over the same period {2,14,18}. In those who developed breakthrough varicella postvaccination, the majority experienced mild disease, with the median of the maximum total number of lesions <50. The severity of reported breakthrough varicella, as measured by number of lesions and maximum temperature, appeared not to increase with time since vaccination.
Among a subset of vaccinees who were actively followed in these later trials for up to 10 years postvaccination, 95 individuals were exposed to an unvaccinated individual with wild-type varicella in a household setting. There were no reports of breakthrough varicella in 92% (87/95) of exposed children, while 8% (8/95) reported a mild form of varicella (maximum total number of lesions <50; observed range, 10 to 34). This represents an estimated efficacy of 90% (95% CI, 82%, 96%) based on the historical attack rate of 87% following household exposure to varicella in unvaccinated individuals in the calculation of efficacy.
Two-Dose Regimen in Children
In a clinical trial, a total of 2216 children 12 months to 12 years of age with a negative history of varicella were randomized to receive either 1 dose of VARIVAX (n=1114) or 2 doses of VARIVAX (n=1102) given 3 months apart. Subjects were actively followed for varicella, any varicella-like illness, or herpes zoster and any exposures to varicella or herpes zoster on an annual basis for 10 years after vaccination. Persistence of VZV antibody was measured annually for 9 years. Most cases of varicella reported in recipients of 1 dose or 2 doses of vaccine were mild {15}. The estimated vaccine efficacy for the 10-year observation period was 94% for 1 dose and 98% for 2 doses (p<0.001). This translates to a 3.4-fold lower risk of developing varicella >42 days postvaccination during the 10-year observation period in children who received 2 doses than in those who received 1 dose (2.2% vs. 7.5%, respectively).
Clinical Data in Adolescents and Adults
Two-Dose Regimen in Adolescents and Adults
In early clinical trials, a total of 796 adolescents and adults received 905 to 1230 PFU of attenuated virus per dose of VARIVAX and have been followed for up to six years following 2-dose vaccination. A total of 50 clinical varicella cases were reported >42 days following 2-dose vaccination. Based on passive follow-up, the annual varicella breakthrough event rate ranged from <0.1 to 1.9%. The median of the maximum total number of lesions ranged from 15 to 42 per year.
Although no placebo-controlled trial was carried out in adolescents and adults, the protective efficacy of VARIVAX was determined by evaluation of protection when vaccinees received 2 doses of VARIVAX 4 or 8 weeks apart and were subsequently exposed to varicella in a household setting. Among the subset of vaccinees who were actively followed in these early trials for up to six years, 76 individuals had household exposure to varicella. There were no reports of breakthrough varicella in 83% (63/76) of exposed vaccinees, while 17% (13/76) reported a mild form of varicella. Among 13 vaccinated individuals who developed breakthrough varicella after a household exposure, 62% (8/13) of the cases reported maximum total number of lesions <50, while no individual reported >75 lesions. The attack rate of unvaccinated adults exposed to a single contact in a household has not been previously studied. Utilizing the previously reported historical attack rate of 87% for wild-type varicella following household exposure to varicella among unvaccinated children in the calculation of efficacy, this represents an approximate 80% reduction in the expected number of cases in the household setting.
In later clinical trials, a total of 220 adolescents and adults received 3315 to 9000 PFU of attenuated virus per dose of VARIVAX and have been actively followed for up to six years following 2-dose vaccination. A total of 3 clinical varicella cases were reported >42 days following 2-dose vaccination. Two cases reported <50 lesions and none reported >75. The annual varicella breakthrough event rate ranged from 0 to 1.2%. Among the subset of vaccinees who were actively followed in these later trials for up to five years, 16 individuals were exposed to an unvaccinated individual with wild-type varicella in a household setting. There were no reports of breakthrough varicella among the exposed vaccinees.
There are insufficient data to assess the rate of protective efficacy of VARIVAX against the serious complications of varicella in adults (e.g., encephalitis, hepatitis, pneumonitis) and during pregnancy (congenital varicella syndrome).
14.2 Immunogenicity
In clinical trials, varicella antibodies have been evaluated following vaccination with formulations of VARIVAX containing attenuated virus ranging from 1000 to 50,000 PFU per dose in healthy individuals ranging from 12 months to 55 years of age {2,9}.
One-Dose Regimen in Children
In prelicensure efficacy studies, seroconversion was observed in 97% of vaccinees at approximately 4 to 6 weeks postvaccination in 6889 susceptible children 12 months to 12 years of age. Titers ≥5 gpELISA units/mL were induced in approximately 76% of children vaccinated with a single dose of vaccine at 1000 to 17,000 PFU per dose. Rates of breakthrough disease were significantly lower among children with VZV antibody titers ≥5 gpELISA units/mL compared with children with titers <5 gpELISA units/mL.
Two-Dose Regimen in Children
In a multicenter study, 2216 healthy children 12 months to 12 years of age received either 1 dose of VARIVAX or 2 doses administered 3 months apart. The immunogenicity results are shown in Table 3.
Table 3: Summary of VZV Antibody Responses at 6 Weeks Postdose 1 and 6 Weeks Postdose 2 in Initially Seronegative Children 12 Months to 12 Years of Age (Vaccinations 3 Months Apart) VARIVAX
1-Dose Regimen
(N=1114)VARIVAX
2-Dose Regimen (3 months apart)
(N=1102)6 Weeks Postvaccination (n=892) 6 Weeks Postdose 1 (n=851) 6 Weeks Postdose 2 (n=769) N = Number of subjects vaccinated. n = Number of subjects included in immunogenicity analysis. Seroconversion Rate 98.9% 99.5% 99.9% Percent with VZV Antibody Titer ≥5 gpELISA units/mL 84.9% 87.3% 99.5% Geometric mean titers in gpELISA units/mL (95% CI) 12.0
(11.2, 12.8)12.8
(11.9, 13.7)141.5
(132.3, 151.3)The results from this study and other studies in which a second dose of VARIVAX was administered 3 to 6 years after the initial dose demonstrate significant boosting of the VZV antibodies with a second dose. VZV antibody levels after 2 doses given 3 to 6 years apart are comparable to those obtained when the 2 doses are given 3 months apart.
Two-Dose Regimen in Adolescents and Adults
In a multicenter study involving susceptible adolescents and adults 13 years of age and older, 2 doses of VARIVAX administered 4 to 8 weeks apart induced a seroconversion rate of approximately 75% in 539 individuals 4 weeks after the first dose and of 99% in 479 individuals 4 weeks after the second dose. The average antibody response in vaccinees who received the second dose 8 weeks after the first dose was higher than that in vaccinees who received the second dose 4 weeks after the first dose. In another multicenter study involving adolescents and adults, 2 doses of VARIVAX administered 8 weeks apart induced a seroconversion rate of 94% in 142 individuals 6 weeks after the first dose and 99% in 122 individuals 6 weeks after the second dose.
14.3 Persistence of Immune Response
One-Dose Regimen in Children
In clinical studies involving healthy children who received 1 dose of vaccine, detectable VZV antibodies were present in 99.0% (3886/3926) at 1 year, 99.3% (1555/1566) at 2 years, 98.6% (1106/1122) at 3 years, 99.4% (1168/1175) at 4 years, 99.2% (737/743) at 5 years, 100% (142/142) at 6 years, 97.4% (38/39) at 7 years, 100% (34/34) at 8 years, and 100% (16/16) at 10 years postvaccination.
Two-Dose Regimen in Children
In recipients of 1 dose of VARIVAX over 9 years of follow-up, the geometric mean titers (GMTs) and the percent of subjects with VZV antibody titers ≥5 gpELISA units/mL generally increased. The GMTs and percent of subjects with VZV antibody titers ≥5 gpELISA units/mL in the 2-dose recipients were higher than those in the 1-dose recipients for the first year of follow-up and generally comparable thereafter. The cumulative rate of VZV antibody persistence with both regimens remained very high at year 9 (99.0% for the 1-dose group and 98.8% for the 2-dose group).
Two-Dose Regimen in Adolescents and Adults
In clinical studies involving healthy adolescents and adults who received 2 doses of vaccine, detectable VZV antibodies were present in 97.9% (568/580) at 1 year, 97.1% (34/35) at 2 years, 100% (144/144) at 3 years, 97.0% (98/101) at 4 years, 97.4% (76/78) at 5 years, and 100% (34/34) at 6 years postvaccination.
A boost in antibody levels has been observed in vaccinees following exposure to wild-type varicella, which could account for the apparent long-term persistence of antibody levels in these studies.
14.4 Studies with Other Vaccines
Concomitant Administration with M-M-R II
In combined clinical studies involving 1080 children 12 to 36 months of age, 653 received VARIVAX and M-M-R II concomitantly at separate injection sites and 427 received the vaccines six weeks apart. Seroconversion rates and antibody levels to measles, mumps, rubella, and varicella were comparable between the two groups at approximately six weeks postvaccination.
Concomitant Administration with Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed (DTaP) and Oral Poliovirus Vaccine (OPV)
In a clinical study involving 318 children 12 months to 42 months of age, 160 received an investigational varicella-containing vaccine (a formulation combining measles, mumps, rubella, and varicella in one syringe) concomitantly with booster doses of DTaP and OPV (no longer licensed in the United States). The comparator group of 144 children received M-M-R II concomitantly with booster doses of DTaP and OPV followed by VARIVAX six weeks later. At six weeks postvaccination, seroconversion rates for measles, mumps, rubella, and VZV and the percentage of vaccinees whose titers were boosted for diphtheria, tetanus, pertussis, and polio were comparable between the two groups. Anti-VZV levels were decreased when the investigational vaccine containing varicella was administered concomitantly with DTaP {19}. No clinically significant differences were noted in adverse reactions between the two groups.
Concomitant Administration with PedvaxHIB®
In a clinical study involving 307 children 12 to 18 months of age, 150 received an investigational varicella-containing vaccine (a formulation combining measles, mumps, rubella, and varicella in one syringe) concomitantly with a booster dose of PedvaxHIB [Haemophilus b Conjugate Vaccine (Meningococcal Protein Conjugate)], while 130 received M-M-R II concomitantly with a booster dose of PedvaxHIB followed by VARIVAX 6 weeks later. At six weeks postvaccination, seroconversion rates for measles, mumps, rubella, and VZV, and GMTs for PedvaxHIB were comparable between the two groups. Anti-VZV levels were decreased when the investigational vaccine containing varicella was administered concomitantly with PedvaxHIB {20}. No clinically significant differences in adverse reactions were seen between the two groups.
Concomitant Administration with M-M-R II and COMVAX
In a clinical study involving 822 children 12 to 15 months of age, 410 received COMVAX, M-M-R II, and VARIVAX concomitantly at separate injection sites, and 412 received COMVAX followed by M-M-R II and VARIVAX given concomitantly at separate injection sites, 6 weeks later. At 6 weeks postvaccination, the immune responses for the subjects who received the concomitant doses of COMVAX, M-M-R II, and VARIVAX were similar to those of the subjects who received COMVAX followed 6 weeks later by M-M-R II and VARIVAX with respect to all antigens administered. There were no clinically important differences in reaction rates when the three vaccines were administered concomitantly versus six weeks apart.
-
15 REFERENCES
- CDC: General Recommendations on Immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 55(No. RR-15): 1-47, 2006.
- Weibel, R.E.; et al.: Live Attenuated Varicella Virus Vaccine. Efficacy Trial in Healthy Children. N Engl J Med. 310(22): 1409-1415, 1984.
- Arbeter, A.M.; et al.: Varicella Vaccine Trials in Healthy Children. A Summary of Comparative and Follow-up Studies. Am J Dis Child. 138: 434-438, 1984.
- Weibel, R.E.; et al.: Live Oka/Merck Varicella Vaccine in Healthy Children. Further Clinical and Laboratory Assessment. JAMA. 254(17): 2435-2439, 1985.
- Chartrand, D.M.; et al.: New Varicella Vaccine Production Lots in Healthy Children and Adolescents. Abstracts of the 1988 Inter-Science Conference Antimicrobial Agents and Chemotherapy: 237(Abstract #731).
- Johnson, C.E.; et al.: Live Attenuated Varicella Vaccine in Healthy 12- to 24-Month-Old Children. Pediatrics. 81(4): 512-518, 1988.
- Gershon, A.A.; et al.: Immunization of Healthy Adults with Live Attenuated Varicella Vaccine. J Infect Dis. 158(1): 132-137, 1988.
- Gershon, A.A.; et al.: Live Attenuated Varicella Vaccine: Protection in Healthy Adults Compared with Leukemic Children. J Infect Dis. 161: 661-666, 1990.
- White, C.J.; et al.: Varicella Vaccine (VARIVAX) in Healthy Children and Adolescents: Results From Clinical Trials, 1987 to 1989. Pediatrics. 87(5): 604-610, 1991.
- Rynn L, Cragan J, Correa A. Update on Overall Prevalence of Major Birth Defects Atlanta, 1978-2005. CDC MMWR. 57(01): 1-5, January 11, 2008.
- American College of Obstetricians and Gynecologists Frequently Asked Questions: Miscarriage and Molar Pregnancy, 2011.
- Galea, S.; et al.: The Safety Profile of Varicella Vaccine: A 10-Year Review. J Infect Dis. 197(S2): 165-169, 2008.
- Hammerschlag, M.R.; et al.: Herpes Zoster in an Adult Recipient of Live Attenuated Varicella Vaccine. J Infect Dis. 160(3): 535-537, 1989.
- Guess, H.A.; et al.: Population-Based Studies of Varicella Complications. Pediatrics. 78(suppl): 723-727, 1986.
- Kuter, B.J.; et al.: Ten Year Follow-up of Healthy Children who Received One or Two Injections of Varicella Vaccine. Pediatr Infect Dis J. 23: 132-37, 2004.
- Kuter, B.J.; et al.: Oka/Merck Varicella Vaccine in Healthy Children: Final Report of a 2-Year Efficacy Study and 7-Year Follow-up Studies. Vaccine. 9: 643-647, 1991.
- Bernstein, H.H.; et al.: Clinical Survey of Natural Varicella Compared with Breakthrough Varicella After Immunization with Live Attenuated Oka/Merck Varicella Vaccine. Pediatrics. 92(6): 833-837, 1993.
- Wharton, M.: The Epidemiology of Varicella-zoster Virus Infections. Infect Dis Clin North Am. 10(3):571-581, 1996.
- White, C.J. et al.: Measles, Mumps, Rubella, and Varicella Combination Vaccine: Safety and Immunogenicity Alone and in Combination with Other Vaccines Given to Children. Clin Infect Dis. 24(5): 925-931, 1997.
- Reuman, P.D.; et al.: Safety and Immunogenicity of Concurrent Administration of Measles-Mumps-Rubella-Varicella Vaccine and PedvaxHIB® Vaccines in Healthy Children Twelve to Eighteen Months Old. Pediatr Infect Dis J. 16(7): 662-667, 1997.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
No. 4827/4309 —VARIVAX is supplied as follows:
(1) a box of 10 single-dose vials of lyophilized vaccine (package A), NDC: 0006-4827-00
(2) a box of 10 vials of diluent (package B).Storage
Vaccine Vial
During shipment, maintain the vaccine at a temperature between –58°F and +5°F (–50°C and –15°C). Use of dry ice may subject VARIVAX to temperatures colder than –58°F (–50°C).
Before reconstitution, store the lyophilized vaccine in a freezer at a temperature between –58°F and +5°F (–50°C and –15°C). Any freezer (e.g., chest, frost-free) that reliably maintains an average temperature between –58°F and +5°F (–50°C and –15°C) and has a separate sealed freezer door is acceptable for storing VARIVAX. Routine defrost cycling of a frost-free freezer is acceptable.
VARIVAX may be stored at refrigerator temperature (36°F to 46°F, 2°C to 8°C) for up to 72 continuous hours prior to reconstitution. Vaccine stored at 2°C to 8°C which is not used within 72 hours of removal from +5°F (–15°C) storage should be discarded.
Before reconstitution, protect from light.
DISCARD IF RECONSTITUTED VACCINE IS NOT USED WITHIN 30 MINUTES.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Discuss the following with the patient:
- Question the patient, parent, or guardian about reactions to previous vaccines.
- Provide a copy of the patient information (PPI) located at the end of this insert and discuss any questions or concerns.
- Inform patient, parent, or guardian that vaccination with VARIVAX may not result in protection of all healthy, susceptible children, adolescents, and adults.
- Inform female patients to avoid pregnancy for three months following vaccination.
- Inform patient, parent, or guardian of the benefits and risks of VARIVAX.
- Instruct patient, parent, or guardian to report any adverse reactions or any symptoms of concern to their healthcare professional.
The U.S. Department of Health and Human Services has established a Vaccine Adverse Event Reporting System (VAERS) to accept all reports of suspected adverse events after the administration of any vaccine. For information or a copy of the vaccine reporting form, call the VAERS toll-free number at 1-800-822-7967, or report online at www.vaers.hhs.gov.
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
Patient Information about
VARIVAX® (pronounced "VAR ih vax")
Generic name: Varicella Virus Vaccine LiveThis is a summary of information about VARIVAX®. You should read it before you or your child get the vaccine. If you have any questions about the vaccine after reading this leaflet, you should ask your healthcare professional. This is a summary only. It does not take the place of talking about VARIVAX with your doctor, nurse, or other healthcare professional. Only your healthcare professional can decide if VARIVAX is right for you or your child.
What is VARIVAX and how does it work?
VARIVAX is also known as Varicella Virus Vaccine Live. It is a live virus vaccine that is given as a shot. It is meant to help prevent chickenpox. Chickenpox is sometimes called varicella (pronounced VAR ih sell a).
VARIVAX contains a weakened form of chickenpox virus.
VARIVAX works by helping the immune system protect you or your child from getting chickenpox.
VARIVAX may not protect everyone who gets it.
VARIVAX does not treat chickenpox once you or your child have it.
What do I need to know about chickenpox?
Chickenpox is an illness that occurs most often in children who are 5 to 9 years old. It can be passed to others. The illness can include headache, fever, and general discomfort. Then an itchy rash occurs, which can turn into blisters. The most common complication is that the blisters can get infected. Less common but very serious complications can occur. These include pneumonia, inflammation of the brain, Reye syndrome (which affects the liver and the brain), and death. Severe disease and serious complications are more likely to occur in adolescents and adults.
Who should not get VARIVAX?
Do not get VARIVAX if you or your child:
- are allergic to any of its ingredients. (This includes gelatin or neomycin. See the ingredient list at the end of this leaflet.)
- have a weakened immune system, such as an immune deficiency, an inherited immune disorder, leukemia, lymphoma, or HIV/AIDS.
- take high doses of steroids by mouth or in a shot.
- have active tuberculosis that is not treated.
- have a fever.
- are pregnant or plan to get pregnant within the next three months.
What should I tell my healthcare professional before getting VARIVAX?
Tell your healthcare professional if you or your child:
- have or have had any medical problems.
- have received blood or plasma transfusions or human serum globulin within the last 5 months.
- take any medicines. (This includes non-prescription medicines and dietary supplements.)
- have any allergies. (This includes allergies to neomycin or gelatin.)
- had an allergic reaction to any other vaccine.
- are pregnant or plan to become pregnant within the next three months.
- are breast-feeding.
How is VARIVAX given?
VARIVAX is given as a shot to people who are 12 months old or older. If your child is 12 months to 12 years old and your doctor gives a second dose, the second dose must be given at least 3 months after the first shot.
A second dose should be given to those who first get the vaccine when they are 13 years old or older. This second dose should be given 4 to 8 weeks after the first dose.
Your doctor or healthcare professional will use the official recommendations to decide the number of shots needed and when to get them.
If a dose is missed, your healthcare professional will let you know when you should have it.
What should you or your child avoid when getting VARIVAX?
Do not take aspirin or aspirin-containing products for 6 weeks after getting VARIVAX.
In rare circumstances, it is possible to catch chickenpox, including severe chickenpox, from a person who has been vaccinated with VARIVAX. This may occur in persons who have not previously been vaccinated or had chickenpox, as well as persons who fall into one of the following categories:
- people who have a weakened immune system.
- pregnant women who have never had chickenpox.
- newborn babies whose mothers have never had chickenpox.
- newborn babies born at less than 28 weeks of pregnancy.
Whenever possible, individuals who have been vaccinated with VARIVAX should attempt to avoid close contact for up to six weeks following the vaccination, with anyone who falls into one of the categories above. Tell your doctor or healthcare professional if you or your child expect to have close contact with someone who falls into one of these groups.
What are the possible side effects of VARIVAX?
The most common side effects reported after taking VARIVAX are:
- Fever
- Pain, swelling, itching, or redness at the site of the shot
- Chickenpox-like rash on the body or at the site of the shot
- Irritability
Other less common side effects have also been reported.
- Tingling of the skin
- Shingles (herpes zoster)
Tell your healthcare professional if you have any of the following problems within a short time after getting VARIVAX because they may be signs of an allergic reaction:
- Shortness of breath or wheezing
- Rash or hives
Other side effects have been reported. Some of them were serious. These include bruising more easily than normal; red or purple, flat, pinhead spots under the skin; severe paleness; difficulty walking; severe skin disorders; skin infection; and chickenpox. Rarely, swelling of the brain (encephalitis), stroke, inflammation of the coverings of the brain and spinal cord (meningitis), inflammation of the lungs (known as pneumonia or pneumonitis), and seizures with or without a fever have been reported. It is not known if these rare side effects are related to the vaccine.
Your doctor has a more complete list of side effects for VARIVAX.
Tell your doctor or healthcare professional if you or your child have any new or unusual symptoms after getting VARIVAX.
Report the following to your doctor or your child's doctor:
- any adverse reactions following vaccination
- exposure to VARIVAX during pregnancy
- exposure to VARIVAX during the 3 months before getting pregnant.
You may also report these events to Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231, or directly to the Vaccine Adverse Event Reporting System (VAERS). The VAERS toll-free number is 1-800-822-7967 or report online to www.vaers.hhs.gov.
What are the ingredients of VARIVAX?
Active Ingredient: a weakened form of chickenpox virus.
Inactive Ingredients: sucrose, hydrolyzed gelatin, sodium chloride, monosodium L-glutamate, sodium phosphate dibasic, potassium phosphate monobasic, potassium chloride, residual components of MRC-5 cells including DNA and protein, sodium phosphate monobasic, EDTA, neomycin, fetal bovine serum.
What else should I know about VARIVAX?
This leaflet summarizes important information about VARIVAX.
If you would like more information, talk to your healthcare professional, visit the web site at www.merckvaccines.com, or call 1-800-Merck-90.
- SPL UNCLASSIFIED SECTION
-
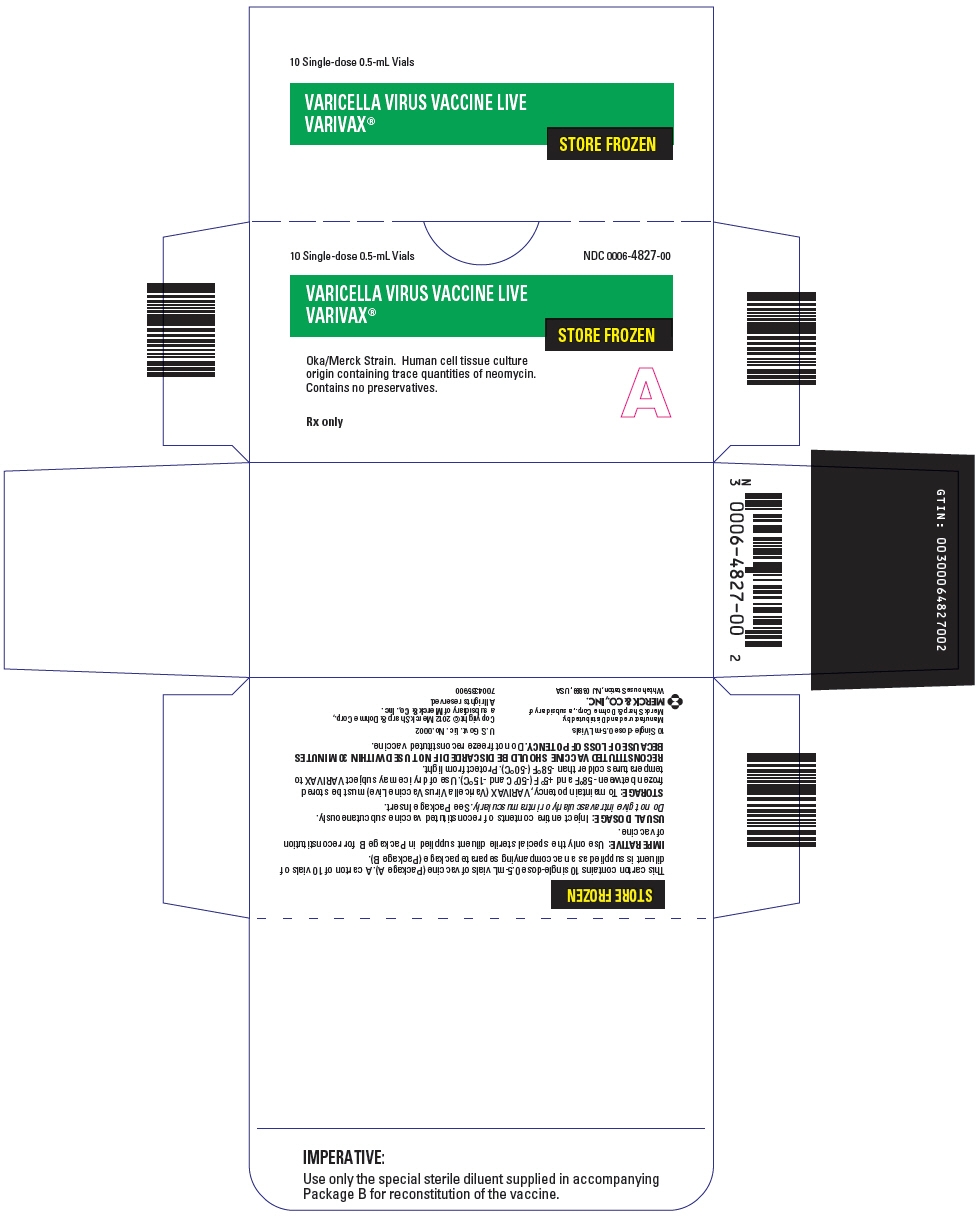
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 1 Single Dose 0.5 mL Vial Carton
NDC: 0006-4826-00
1 Single-dose 0.5-mL Vial
VARICELLA VIRUS VACCINE
LIVE
VARIVAX®STORE FROZEN
Oka/Merck Strain. Human cell
tissue culture origin containing
trace quantities of neomycin.
Contains no preservatives.Rx only
A

-
PRINCIPAL DISPLAY PANEL - 10 Single Dose 0.5 mL Vial Carton
10 Single-dose 0.5-mL Vials
NDC: 0006-4827-00VARICELLA VIRUS VACCINE LIVE
VARIVAX®STORE FROZEN
Oka/Merck Strain. Human cell tissue culture
origin containing trace quantities of neomycin.
Contains no preservatives.Rx only
A
-
INGREDIENTS AND APPEARANCE
VARIVAX
varicella virus vaccine live injection, powder, lyophilized, for suspensionProduct Information Product Type VACCINE Item Code (Source) NDC: 0006-4826 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VARICELLA-ZOSTER VIRUS STRAIN OKA/MERCK LIVE ANTIGEN (UNII: GPV39ZGD8C) (VARICELLA-ZOSTER VIRUS STRAIN OKA/MERCK LIVE ANTIGEN - UNII:GPV39ZGD8C) VARICELLA-ZOSTER VIRUS STRAIN OKA/MERCK LIVE ANTIGEN 1350 [PFU] in 0.5 mL Inactive Ingredients Ingredient Name Strength EDETIC ACID (UNII: 9G34HU7RV0) ALBUMIN BOVINE (UNII: 27432CM55Q) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) 12.5 mg in 0.5 mL MONOSODIUM GLUTAMATE (UNII: W81N5U6R6U) 0.5 mg in 0.5 mL NEOMYCIN (UNII: I16QD7X297) POTASSIUM CHLORIDE (UNII: 660YQ98I10) 0.08 mg in 0.5 mL POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) 0.08 mg in 0.5 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 3.2 mg in 0.5 mL SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) 0.45 mg in 0.5 mL SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) SUCROSE (UNII: C151H8M554) 25 mg in 0.5 mL Product Characteristics Color YELLOW (clear, colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-4826-00 1 in 1 CARTON 1 NDC: 0006-4826-01 0.5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103552 03/17/1995 VARIVAX
varicella virus vaccine live injection, powder, lyophilized, for suspensionProduct Information Product Type VACCINE Item Code (Source) NDC: 0006-4827 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VARICELLA-ZOSTER VIRUS STRAIN OKA/MERCK LIVE ANTIGEN (UNII: GPV39ZGD8C) (VARICELLA-ZOSTER VIRUS STRAIN OKA/MERCK LIVE ANTIGEN - UNII:GPV39ZGD8C) VARICELLA-ZOSTER VIRUS STRAIN OKA/MERCK LIVE ANTIGEN 1350 [PFU] in 0.5 mL Inactive Ingredients Ingredient Name Strength EDETIC ACID (UNII: 9G34HU7RV0) ALBUMIN BOVINE (UNII: 27432CM55Q) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) 12.5 mg in 0.5 mL MONOSODIUM GLUTAMATE (UNII: W81N5U6R6U) 0.5 mg in 0.5 mL NEOMYCIN (UNII: I16QD7X297) POTASSIUM CHLORIDE (UNII: 660YQ98I10) 0.08 mg in 0.5 mL POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) 0.08 mg in 0.5 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 3.2 mg in 0.5 mL SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) 0.45 mg in 0.5 mL SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) SUCROSE (UNII: C151H8M554) 25 mg in 0.5 mL Product Characteristics Color YELLOW (clear, colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-4827-00 10 in 1 CARTON 1 NDC: 0006-4827-01 0.5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103552 03/17/1995 Labeler - Merck Sharp & Dohme Corp. (001317601) Establishment Name Address ID/FEI Business Operations Merck Sharp & Dohme Corp. 002387926 API MANUFACTURE(0006-4826, 0006-4827) , MANUFACTURE(0006-4826, 0006-4827) , ANALYSIS(0006-4826, 0006-4827) , PACK(0006-4826, 0006-4827) Establishment Name Address ID/FEI Business Operations Merck Sharp & Dohme Corp. 621573075 MANUFACTURE(0006-4826, 0006-4827) , API MANUFACTURE(0006-4826, 0006-4827) , ANALYSIS(0006-4826, 0006-4827) Establishment Name Address ID/FEI Business Operations Merck Sharp & Dohme Corp. 101740835 PACK(0006-4826, 0006-4827)
Trademark Results [VARIVAX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VARIVAX 73509696 1340022 Live/Registered |
MERCK & CO., INC. 1984-11-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.