HYDROCORTISONE IODOQUINOL- hydrocortisone and iodoquinol cream
HYDROCORTISONE IODOQUINOL by
Drug Labeling and Warnings
HYDROCORTISONE IODOQUINOL by is a Prescription medication manufactured, distributed, or labeled by Syntenza Pharmaceuticals LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each gram of Hydrocortisone 1% – Iodoquinol 1% Cream contains 10 mg of hydrocortisone and 10 mg of iodoquinol in a greaseless base of cetyl alcohol, glyceryl monostearate SE, isopropyl myristate, lanolin alcohol, mineral oil, polyoxyl 40 stearate, polysorbate 20, polysorbate 60, propylene glycol, purified water, sorbic acid, and sorbitan monostearate. Paraben free.
Chemically, hydrocortisone is [Pregn-4-ene-3,20-dione, 11, 17, 21-trihydroxy-,(11ß)-] with the molecular formula C21H30O5 and is represented by the following structural formula:
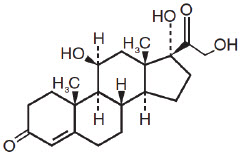
and iodoquinol, 5,7-diiodo-8-quinolinol (C9H5I2NO) is represented by the following structure:
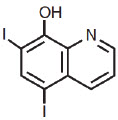
Hydrocortisone is an anti-inflammatory and antipruritic agent, while iodoquinol is an antifungal and antibacterial agent.
-
CLINICAL PHARMACOLOGY
Hydrocortisone has anti-inflammatory, antipruritic and vasoconstrictor properties. The mechanism of anti-inflammatory activity is unclear. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Iodoquinol has both antifungal and antibacterial properties.
Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Hydrocortisone can be absorbed from normal intact skin. Inflammation and/or other inflammatory disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids.
Once absorbed through the skin, hydrocortisone is metabolized in the liver and most body tissues to hydrogenated and degraded forms such as tetrahydrocortisone and tetrahydrocortisol. These are excreted in the urine, mainly conjugated as glucuronides, together with a very small proportion of unchanged hydrocortisone.
There are no data available regarding the percutaneous absorption of iodoquinol; however, following oral administration, 3-5% of the dose was recovered in the urine as a glucuronide.
-
INDICATIONS & USAGE
INDICATIONS AND USAGE Based on a review of a related drug by the National Research Council and subsequent FDA classification for that drug, the indications are as follows: "Possibly" Effective: Contact or atopic dermatitis; impetiginized eczema; nummular eczema; infantile eczema; endogenous chronic infectious dermatitis; stasis dermatitis; pyoderma; nuchal eczema and chronic eczematoid otitis externa; acne urticata; localized or disseminated neurodermatitis; lichen simplex chronicus; anogenital pruritus (vulvae, scroti, ani); folliculitis, bacterial dermatoses; mycotic dermatoses such as tinea (capitis, cruris, corporis, pedis); moniliasis, intertrigo. Final classification of the less-than-effective indications requires further investigation. - CONTRAINDICATIONS
-
WARNINGS
FOR EXTERNAL USE ONLY. Keep away from eyes. Keep out of reach of children. Keep tube tightly closed.
If irritation develops, the use of Hydrocortisone 1% – Iodoquinol 1% Cream should be discontinued and appropriate therapy instituted. Staining of the skin, hair and fabrics may occur. If extensive areas are treated or if the occlusive technique is used, the possibility exists of increased systemic absorption of the corticosteroid, and suitable precautions should be taken. Children may absorb proportionally larger amounts of topical corticosteroids and thus be more susceptible to systemic toxicity. Parents of pediatric patients should be advised not to use tight-fitting diapers or plastic pants on a child being treated in the diaper area, as these garments may constitute occlusive dressings. Iodoquinol may be absorbed through the skin and interfere with thyroid function tests. If such tests are contemplated, wait at least one month after discontinuance of therapy to perform these tests. The ferric chloride test for phenylketonuria (PKU) can yield a false positive result if iodoquinol is present in the diaper or urine.
Prolonged use may result in overgrowth of non-susceptible organisms requiring appropriate therapy.
-
PRECAUTIONS
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of hydrocortisone or iodoquinol.
In vitro studies to determine mutagenicity with hydrocortisone have revealed negative results. Mutagenicity studies have not been conducted with iodoquinol.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproductive studies have not been conducted with Hydrocortisone 1% – Iodoquinol 1% Cream. It is not known whether Hydrocortisone 1% – Iodoquinol 1% Cream can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Hydrocortisone 1% – Iodoquinol 1% Cream should be given to a pregnant woman only if clearly needed.
-
ADVERSE REACTIONS
The following local adverse reactions are reported infrequently with topical corticosteroids. These reactions are listed in an approximate decreasing order of occurrence:
Burning Perioral dermatitis Itching Allergic contact dermatitis Irritation Maceration of the skin Dryness Secondary infection Folliculitis Skin atrophy Hypertrichosis Striae Acneiform eruptions Miliaria Hypopigmentation - DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Hydrocortisone 1% – Iodoquinol 1% Cream is available as follows: 1 oz. tube (NDC: 72056-040-64)
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 28.4 g Tube Carton
-
INGREDIENTS AND APPEARANCE
HYDROCORTISONE IODOQUINOL
hydrocortisone and iodoquinol creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 72056-040 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 10 mg in 1 g IODOQUINOL (UNII: 63W7IE88K8) (IODOQUINOL - UNII:63W7IE88K8) IODOQUINOL 10 mg in 1 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Cetyl Alcohol (UNII: 936JST6JCN) Polysorbate 20 (UNII: 7T1F30V5YH) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72056-040-64 1 in 1 CARTON 10/05/2018 1 28.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 10/05/2018 Labeler - Syntenza Pharmaceuticals LLC (080999747)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
