HYDASE- hyaluronidase injection, solution
Hydase by
Drug Labeling and Warnings
Hydase by is a Prescription medication manufactured, distributed, or labeled by Akorn, Inc., Akorn, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HYDASE™ Bovine safely and effectively. See full prescribing information for HYDASE.
HYDASE™ (hyaluronidase injection) Bovine, for infiltration use, for interstitial use, for intramuscular use, for intraocular use, for retrobulbar use, for soft tissue use, and for subcutaneous use
Initial U.S. Approval: 2005INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- See Full Prescribing Information for all approved routes of administration. (2.1)
- Absorption and Dispersion of Injected Drugs
Absorption and dispersion of other injected drugs may be enhanced by adding 50 to 300 Units, most typically 150 Units of Hydase™, to the injection solution. (2.2) - Hypodermoclysis
Insert needle with aseptic precautions. With tip lying free and movable between skin and muscle, begin clysis; fluid should start in readily without pain or lump. Then inject Hydase™ into rubber tubing close to needle. (2.3) - Subcutaneous Urography
The subcutaneous route of administration of urographic contrast media is indicated when intravenous administration cannot be successfully accomplished, particularly in infants and small children. With the patient prone, 75 Units of Hydase™ is injected subcutaneously over each scapula, followed by injection of the contrast medium at the same sites. (2.4)
DOSAGE FORMS AND STRENGTHS
Injection: 150 USP Units/mL in single-dose vials (3)
CONTRAINDICATIONS
Hypersensitivity (4)
ADVERSE REACTIONS
The most frequently reported adverse reactions have been local injections site reactions. Allergic reactions (urticarial, angioedema) anaphylactic-like reactions have been reported, rarely. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Akorn, Inc. at 1-800-932-5676, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Furosemide, the benzodiazepines and phenytoin are incompatible with hyaluronidase. (7.1)
- Hyaluronidase should not be used to enhance the absorption and dispersion of dopamine and/or alpha agonist drugs. (7.2)
- Local Anesthetic: Hyaluronidase hastens the onset and shortens duration of effect, increases incidence of systemic reactions. (7.3)
- Large doses of salicylated, cortisone, ACTH, estrogens or antihistamines may require larger amounts of hydaluronidase for equivalent dispensing effect. (7.4)
USE IN SPECIFIC POPULATIONS
Pediatric Use: The dosage of subcutaneous fluids administered is dependent upon the age, weight, and clinical condition of the patient. For premature infants or during the neonatal period, the daily dosage should not exceed 25 mL/kg of body weight, and the rate of administration should not be greater than 2 mL per minute. Special care must be taken in pediatric patients to avoid over hydration by controlling the rate and total volume of the infusion (2.1, 8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2015
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Absorption and Dispersion of Injected Drugs
1.2 Hypodermoclysis
1.3 Subcutaneous Urography
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Absorption and Dispersion of Subcutaneously Injected Drugs
2.3 Hypodermoclysis
2.4 Subcutaneous Urography
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Spread of Localized Injection
5.2 Ocular Damage
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Incompatibilities
7.2 Drug-Specific Precautions
7.3 Local Anesthetics
7.4 Salicylates, Cortisone, ACTH, Estrogens or Antihistamines
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Important Precautions Regarding Hydase™
17.2 What Patients Should Know About Adverse Reactions
17.3 Patients Should Inform Their Doctors If Taking Other Medications
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Do not administer Hydase intravenously because hyaluronidase is enzyme is rapidly inactivated with intravenous administration.
Hydase™ may be administered for infiltration use, interstitial use, intramuscular use, intraocular use, retrobulbar use, soft tissue use and subcutaneous use.
Visually inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever the solution and container permit.
Always use aseptic precautions.
2.2 Absorption and Dispersion of Subcutaneously Injected Drugs
Absorption and dispersion of other injected drugs may be enhanced by adding 50 to 300 Units, most typically 150 Units of hyaluronidase, to the injection solution.
It is recommended that appropriate references be consulted regarding physical or chemical incompatibilities before adding Hydase™ to a solution containing another drug.
2.3 Hypodermoclysis
Insert needle with aseptic precautions. With tip lying free and movable between skin and muscle, begin clysis; fluid should start in readily without pain or lump. Then inject Hydase™ (hyaluronidase injection) into rubber tubing close to needle.
An alternate method is to inject Hydase™ under skin prior to clysis. 150 Units will facilitate absorption of 1,000 mL or more of solution. As with all parenteral fluid therapy, observe effect closely, with same precautions for restoring fluid and electrolyte balance as in intravenous injections. The dose, the rate of injection, and the type of solution (saline, glucose, Ringer's, etc.) must be adjusted carefully to the individual patient. When solutions devoid of inorganic electrolytes are given by hypodermoclysis, hypovolemia may occur. This may be prevented by using solutions containing adequate amounts of inorganic electrolytes and/or controlling the volume and speed of administration.
Hydase™ may be added to small volumes of solution (up to 200 mL), such as small clysis for infants or solutions of drugs for subcutaneous injection. For infants and children less than 3 years old, the volume of a single clysis should be limited to 200 mL; and in premature infants or during the neonatal period, the daily dosage should not exceed 25 mL/kg of body weight; the rate of administration should not be greater than 2 mL per minute. For older patients, the rate and volume of administration should not exceed those employed for intravenous infusion.
2.4 Subcutaneous Urography
The subcutaneous route of administration of urographic contrast media is indicated when intravenous administration cannot be successfully accomplished, particularly in infants and small children. With the patient prone, 75 Units of Hydase™ (hyaluronidase injection) is injected subcutaneously over each scapula, followed by injection of the contrast medium at the same sites.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity
Hydase™ contraindicated in patients with known hypersensitivity to hyaluronidase or any other ingredient in the formulation. A preliminary skin test for hypersensitivity to Hydase™ can be performed. The skin test is made by an intradermal injection of approximately 0.02 mL (3 Units) of a 150 Unit/mL solution [see Dosage and Administration (2)]. A positive reaction consists of a wheal with pseudopods appearing within five minutes and persisting for 20 to 30 minutes and accompanied by localized itching. Transient vasodilation at the site of the test, i.e., erythema, is not a positive reaction.
Discontinue Hydase™ if sensitization occurs.
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
The most frequently reported adverse experiences have been local injection site reactions.
Hyaluronidase has been reported to enhance the adverse events associated with co-administered drug products. Edema has been reported most frequently in association with hypodermoclysis.
Allergic reactions (urticaria, angioedema) have been reported in less than 0.1% of patients receiving hyaluronidase. Anaphylactic-like reactions following retrobulbar block or intravenous injections have occurred, rarely.
-
7 DRUG INTERACTIONS
It is recommended that appropriate references be consulted regarding physical or chemical incompatibilities before adding Hydase™ to a solution containing another drug.
7.1 Incompatibilities
Furosemide, the benzodiazepines and phenytoin have been found to be incompatible with hyaluronidase.
7.2 Drug-Specific Precautions
Hyaluronidase should not be used to enhance the absorption and dispersion of dopamine and/or alpha agonist drugs.
When considering the administration of any other drug with hyaluronidase, it is recommended that appropriate references first be consulted to determine the usual precautions for the use of the other drug.
7.3 Local Anesthetics
When hyaluronidase is added to a local anesthetic agent, it hastens the onset of analgesia and tends to reduce the swelling caused by local infiltration, but the wider spread of the local anesthetic solution increases its absorption; this shortens its duration of action and tends to increase the incidence of systemic reaction.
7.4 Salicylates, Cortisone, ACTH, Estrogens or Antihistamines
Patients receiving large doses of salicylates, cortisone, ACTH, estrogens or antihistamines may require larger amounts of hyaluronidase for equivalent dispersing effect, since these drugs apparently render tissues partly resistant to the action of hyaluronidase.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
No adequate and well controlled animal studies have been conducted with Hydase™ to determine reproductive effects. Hydase™ should be used during pregnancy only if clearly needed.
8.2 Labor and Delivery
Administration of hyaluronidase during labor was reported to cause no complications: no increase in blood loss or differences in cervical trauma were observed. It is not known whether hyaluronidase has an effect on the later growth, development, and functional maturation of the infant.
8.3 Nursing Mothers
It is not known whether hyaluronidase is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when hyaluronidase is administered to a nursing woman.
8.4 Pediatric Use
Hyaluronidase may be added to small volumes of solution (up to 200 mL), such as small clysis for infants or solutions of drugs for subcutaneous injection. The potential for chemical or physical incompatibilities should be kept in mind [see Dosage and Administration (2)].
For infants and children less than 3 years old, the volume of a single clysis should be limited to 200 mL; and in premature infants or during the neonatal period, the daily dosage should not exceed 25 mL/kg of body weight; the rate of administration should not be greater than 2 mL per minute. For older patients, the rate and volume of administration should not exceed those employed for intravenous infusion.
During hypodermoclysis, special care must be taken in pediatric patients to avoid overhydration by controlling the rate and total volume of the clysis [see Dosage and Administration (2.1)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Hydase™ (hyaluronidase injection) is a preparation of purified bovine testicular hyaluronidase, a protein enzyme. The exact chemical structure of this enzyme is unknown.
Hydase™ (hyaluronidase injection) is supplied as sterile, colorless, odorless, ready for use solution. Each vial contains 150 USP units of hyaluronidase per mL calcium chloride (0.4 mg), edetate disodium (1 mg), sodium chloride (8.5 mg), monobasic sodium phosphate buffer, sodium hydroxide to adjust the pH, and sterile water.
Hydase™ has an approximate pH of 6.9 and an osmolality of 275 to 305 mOsm.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Hyaluronidase is a spreading or diffusing substance which modifies the permeability of connective tissue through the hydrolysis of hyaluronic acid, a polysaccharide found in the intercellular ground substance of connective tissue, and of certain specialized tissues, such as the umbilical cord and vitreous humor. Hyaluronic acid is also present in the capsules of type A and C hemolytic streptococci. Hyaluronidase hydrolyzes hyaluronic acid by splitting the glucosaminidic bond between C1 of the glucosamine moiety and C4 of glucuronic acid. This temporarily decreases the viscosity of the cellular cement and promotes diffusion of injected fluids or of localized transudates or exudates, thus facilitating their absorption.
Hyaluronidase cleaves glycosidic bonds of hyaluronic acid and, to a variable degree, some other acid mucopolysaccharides of the connective tissue. The activity is measured in vitro by monitoring the decrease in the amount of an insoluble serum albumin-hyaluronic acid complex as the enzyme cleaves the hyaluronic acid component.
12.2 Pharmacodynamics
When no spreading factor is present, material injected subcutaneously spreads very slowly, but hyaluronidase causes rapid spreading, provided local interstitial pressure is adequate to furnish the necessary mechanical impulse. Such an impulse is normally initiated by injected solutions.
The rate of diffusion is proportionate to the amount of enzyme, and the extent is proportionate to the volume of solution.
12.3 Pharmacokinetics
Knowledge of the mechanisms involved in the disappearance of injected hyaluronidase is limited. It is known, however, that the blood of a number of mammalian species brings about the inactivation of hyaluronidase. Studies have demonstrated that hyaluronidase is antigenic; repeated injections of relatively large amounts of this enzyme may result in the formation of neutralizing anti-bodies. The reconstitution of the dermal barrier removed by intradermal injection of hyaluronidase (20, 2, 0.2, 0.02, and 0.002 Units/mL) to adult humans indicated that at 24 hours the restoration of the barrier is incomplete and inversely related to the dosage of enzyme; at 48 hours the barrier is completely restored in all treated areas.
Results from an experimental study, in humans that evaluated the influence of hyaluronidase in bone repair support the conclusion that this enzyme alone does not deter bone healing when given at the usual clinical dosage.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to assess the carcinogenic or mutagenic potential of hyaluronidase. Hyaluronidase is found in most tissues of the body.
Long-term animal studies have not been performed to assess whether hyaluronidase impaired fertility; however, it has been reported that testicular degeneration may occur with the production of organ-specific antibodies against this enzyme following repeated injections. Human studies on the effect of intravaginal hyaluronidase in sterility due to oligospermia indicated that hyaluronidase may have aided conception.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Hydase™ (hyaluronidase injection) Bovine is supplied sterile as 150 units/mL bovine of hyaluronidase in a single-dose glass vial containing 1 mL.
NDC: 17478-560-01 Package of 1 vial.
NDC: 17478-560-06 Package of 6 vials.
NDC: 17478-560-10 Package of 10 vials.Not recommended for IV Use.
-
17 PATIENT COUNSELING INFORMATION
17.1 Important Precautions Regarding Hydase™
Instruct patient that Hydase™ is being used to increase the dispersion and absorption of fluids or other injected drugs, as appropriate to the intended use.
17.2 What Patients Should Know About Adverse Reactions
The most frequently reported adverse reactions have been mild local injection site reactions such as redness, swelling, itching, or pain.
Anaphylactic-like reactions, and allergic reactions, such as hives, have been reported rarely in patients receiving hyaluronidases.
17.3 Patients Should Inform Their Doctors If Taking Other Medications
You may not receive furosemide, the benzodiazepines, phenytoin, dopamine and/or alpha agonists with Hydase™. These medications have been found to be incompatible with hyaluronidase.
If you are taking salicylates (e.g., aspirin), steroids (e.g., cortisone or estrogens) or antihistamines your doctor may need to prescribe larger amounts of hyaluronidase for equivalent dispersing effect.
AKORN
Manufactured by: Akorn, Inc.
Lake Forest, IL 60045
HD00N Rev. 10/15 -
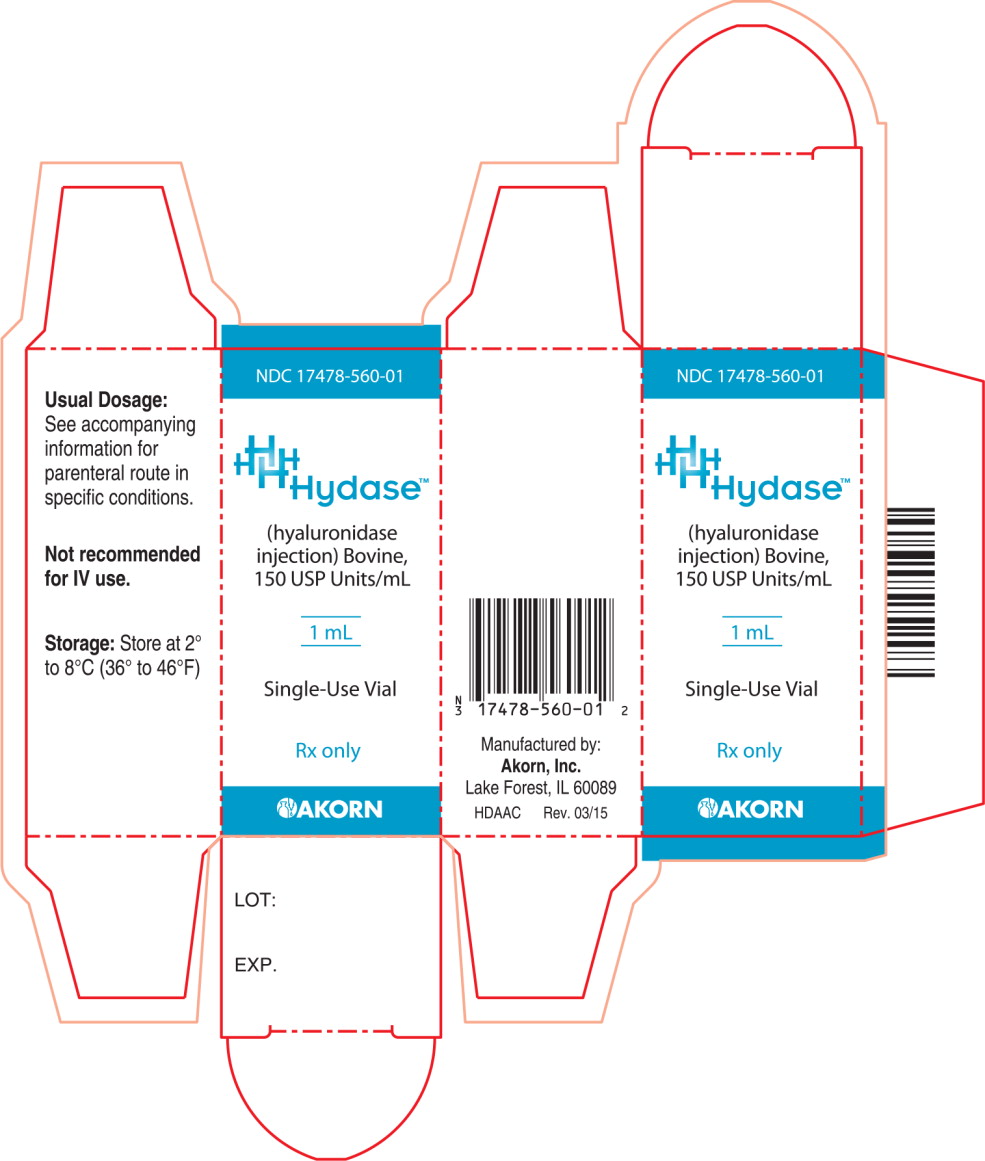
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 17478-560-01
Hydase™
(hyaluronidase
injection) Bovine,
150 USP Units/mL
1 mL
Single-Use Vial
Rx only
Not recommended
for IV use.
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 17478-560-01
Hydase™
(hyaluronidase
injection) Bovine,
150 USP Units/mL
1 mL
Single-Use Vial
Rx only
Akorn Logo
-
INGREDIENTS AND APPEARANCE
HYDASE
hyaluronidase injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17478-560 Route of Administration SUBCUTANEOUS, RETROBULBAR, INTRAMUSCULAR, INTRAOCULAR, INFILTRATION, INTERSTITIAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Hyaluronidase (UNII: 8KOG53Z5EM) (Hyaluronidase - UNII:8KOG53Z5EM) Hyaluronidase 150 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength Calcium Chloride (UNII: M4I0D6VV5M) Edetate Disodium (UNII: 7FLD91C86K) Sodium Chloride (UNII: 451W47IQ8X) Sodium Phosphate, Monobasic (UNII: 3980JIH2SW) Sodium Hydroxide (UNII: 55X04QC32I) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17478-560-01 1 in 1 CARTON 11/05/2015 1 1 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 2 NDC: 17478-560-06 6 in 1 CARTON 11/05/2015 2 1 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 3 NDC: 17478-560-10 10 in 1 CARTON 11/05/2015 3 1 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021716 11/05/2015 Labeler - Akorn, Inc. (062649876) Establishment Name Address ID/FEI Business Operations Akorn, Inc 063434679 PACK(17478-560) , LABEL(17478-560) Establishment Name Address ID/FEI Business Operations Akorn, Inc. 155135783 MANUFACTURE(17478-560) , ANALYSIS(17478-560) , STERILIZE(17478-560)
Trademark Results [Hydase]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HYDASE 86813398 not registered Dead/Abandoned |
Akorn, Inc. 2015-11-09 |
 HYDASE 76330151 not registered Dead/Abandoned |
Purified Proteins, Inc. 2001-10-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.