La RochePosay Laboratoire Dermatologique Anthelios 50 Mineral Ultra Light Sunscreen by L'Oreal USA Products Inc / Goodier Cosmetics LP Drug Facts
La RochePosay Laboratoire Dermatologique Anthelios 50 Mineral Ultra Light Sunscreen by
Drug Labeling and Warnings
La RochePosay Laboratoire Dermatologique Anthelios 50 Mineral Ultra Light Sunscreen by is a Otc medication manufactured, distributed, or labeled by L'Oreal USA Products Inc, Goodier Cosmetics LP. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
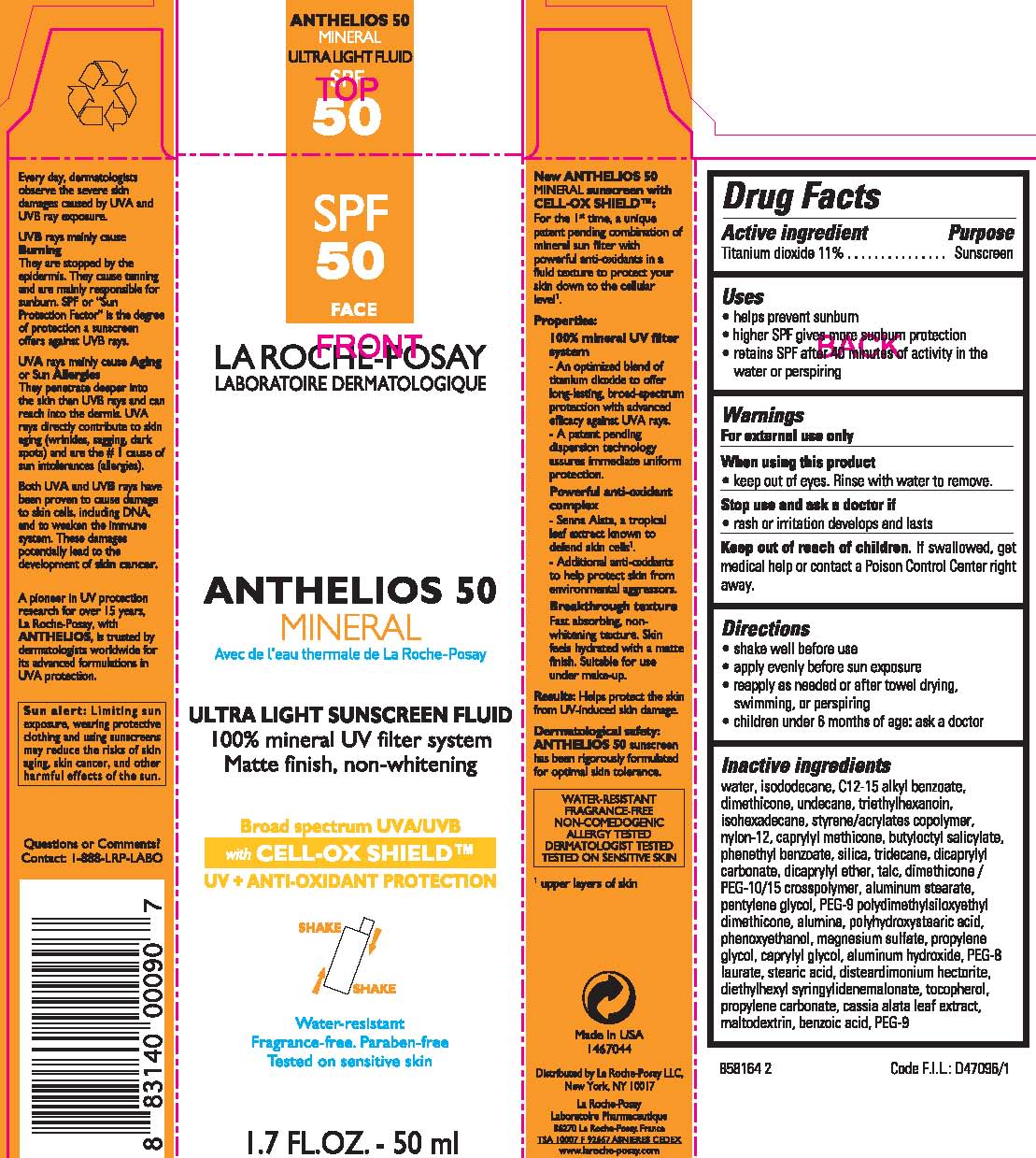
LA ROCHEPOSAY LABORATOIRE DERMATOLOGIQUE ANTHELIOS 50 MINERAL ULTRA LIGHT SUNSCREEN- titanium dioxide lotion
L'Oreal USA Products Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- shake well before use
- apply evenly before sun exposure
- reapply as needed or after towel drying, swimming, or perspiring
- children under 6 months of age: ask a doctor
Inactive ingredients
water, isododecane, C12-15 alkyl benzoate, dimethicone, undecane, triethylhexanoin, isohexadecane, styrene/acrylates copolymer, nylon-12, caprylyl methicone, butyloctyl salicylate, phenethyl benzoate, silica, tridecane, dicaprylyl carbonate, dicaprylyl ether, talc, dimethicone/PEG-10/15 crosspolymer, aluminum stearate, pentylene glycol, PEG-9 polydimethylsiloxyethyl dimethicone, alumina, polyhydroxystearic acid, phenoxyethanol, magnesium sulfate, protpylene glycol, caprylyl glycol, aluminum hydroxide, PEG-8 laurate, stearic acid, disteardimonium hectorite, diethylhexyl syringylidenemalonate, tocopherol, propylene carbonate, cassia alata leaf extract, maltodextrin, benzoic acid, PEG-9
| LA ROCHEPOSAY LABORATOIRE DERMATOLOGIQUE ANTHELIOS 50 MINERAL ULTRA LIGHT SUNSCREEN
titanium dioxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - L'Oreal USA Products Inc (002136794) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Goodier Cosmetics LP | 007317209 | manufacture(49967-090) | |