AFTERA- levonorgestrel tablet
Aftera by
Drug Labeling and Warnings
Aftera by is a Otc medication manufactured, distributed, or labeled by Foundation Consumer Healthcare LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
Sexually transmitted diseases (STDs) alert
This product does not protect against HIV/AIDS or other STDs
Ask a doctor or pharmacist before use if you are taking efavirenz (HIV medication) or rifampin (tuberculosis treatment) or medication for seizures (epilepsy). These medications may reduce the effectiveness of levonorgestrel.
- Directions
-
Other information
- read the instructions, warnings and enclosed product leaflet before use
- this product works mainly by preventing ovulation (egg release). It may also prevent fertilization of a released egg (joining of sperm and egg) or attachment of a fertilized egg to the uterus (implantation).
- do not use if carton is open or tear strip is removed or blister seal is broken or missing
- store at 20-25°C (68-77°F)
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 1.5 mg Tablet Blister Pack Box
NDC: 69536-103-88
Emergency Contraceptive
COMPARE TO PLAN B ONE-STEP®Aftera®
LEVONORGESTREL 1.5 mgReduces chance of pregnancy
after unprotected sex.One Tablet.
One Dose.NOT FOR REGULAR BIRTH CONTROL
1 Tablet
Levonorgestrel
1.5 mg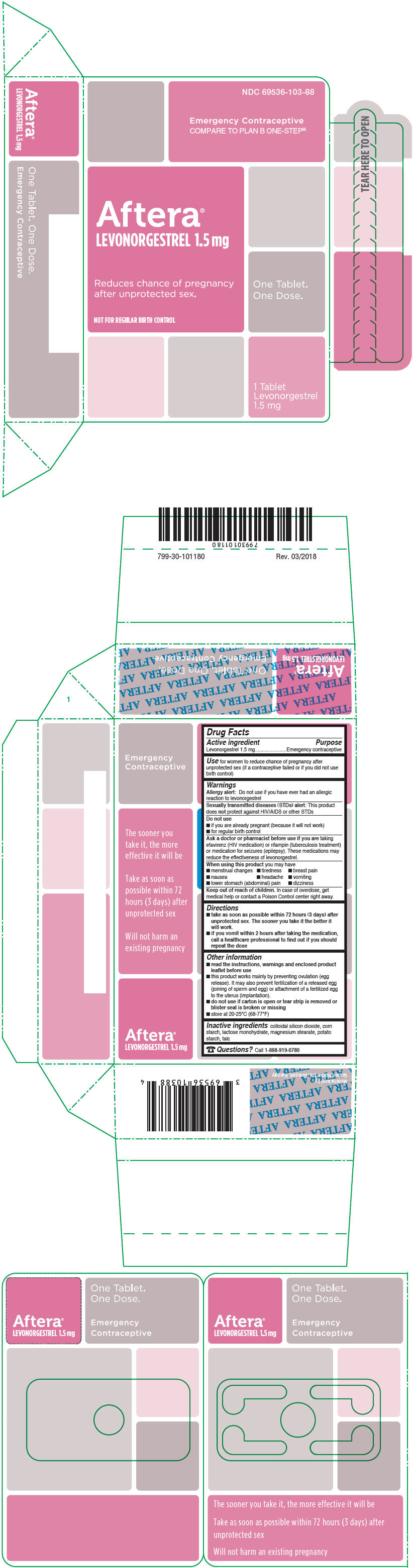
-
INGREDIENTS AND APPEARANCE
AFTERA
levonorgestrel tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69536-103 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVONORGESTREL (UNII: 5W7SIA7YZW) (LEVONORGESTREL - UNII:5W7SIA7YZW) LEVONORGESTREL 1.5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, POTATO (UNII: 8I089SAH3T) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) STARCH, CORN (UNII: O8232NY3SJ) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) Product Characteristics Color WHITE Score no score Shape ROUND Size 8mm Flavor Imprint Code G00 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69536-103-88 1 in 1 BOX, UNIT-DOSE 05/10/2018 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA AUTHORIZED GENERIC NDA021998 05/10/2018 Labeler - Foundation Consumer Healthcare LLC (079675882)
Trademark Results [Aftera]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AFTERA 86066841 4769400 Live/Registered |
TEVA Women's Health, Inc. 2013-09-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.