PILOCARPINE HYDROCHLORIDE solution/ drops
Pilocarpine Hydrochloride by
Drug Labeling and Warnings
Pilocarpine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Sandoz Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Pilocarpine Hydrochloride Ophthalmic Solution safely and effectively. See full prescribing information for Pilocarpine Hydrochloride Ophthalmic Solution.
Pilocarpine Hydrochloride Ophthalmic Solution 1%, 2% and 4%
Initial U.S. Approval: 1974INDICATIONS AND USAGE
Pilocarpine Hydrochloride Ophthalmic Solution is a muscarinic cholinergic agonist indicated for
DOSAGE AND ADMINISTRATION
- Instill one drop in the eye(s) up to four times daily (2).
DOSAGE FORMS AND STRENGTHS
- Solution containing 1% (10 mg/mL), 2% (20 mg/mL) or 4% (40 mg/mL) pilocarpine hydrochloride (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Poor illumination: Exercise caution in night driving and other hazardous occupations in poor illumination (5.1).
- Pre-existing retinal disease: Rare cases of retinal detachment have been reported; a thorough examination of the retina including funduscopy is advised in all patients prior to the initiation of therapy (5.2).
- Iritis: Caution is advised in patients with iritis. (5.3)
- Congenital glaucoma: Caution is advised in pediatric patients with primary congenital glaucoma for control of IOP as cases of a paradoxical increase in IOP have been reported. (5.4)
ADVERSE REACTIONS
Most common adverse reactions are headache/browache, accommodative change, eye irritation, eye pain, blurred vision, and/or visual impairment (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc. at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2011
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Reduction of Elevated Intraocular Pressure (IOP) in Patients with Open-Angle Glaucoma or Ocular Hypertension
2.2 Management of Acute Angle-Closure Glaucoma
2.3 Prevention of Postoperative Elevated IOP Associated with Laser Surgery
2.4 Induction of Miosis
2.5 Use with Other Topical Ophthalmic Medications
2.6 Use in Pediatric Patients
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Poor Illumination
5.2 Pre-existing Retinal Disease
5.3 Iritis
5.4 Primary Congenital Glaucoma
5.5 Contact Lens Wear
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Avoiding Contamination of the Product
17.2 Night Driving
17.3 Accommodative Spasm
17.4 Contact Lens Wear
17.5 Concomitant Topical Ocular Therapy
17.6 Systemic Exposure
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Reduction of Elevated Intraocular Pressure (IOP) in Patients with Open-Angle Glaucoma or Ocular Hypertension
One drop of Pilocarpine Hydrochloride Ophthalmic Solution 1%, 2% or 4% should be applied topically in the eye(s) up to four times daily.
Pilocarpine-naïve patients should be started on the 1% concentration as higher concentrations are often not tolerated initially. The frequency of instillation and concentration of Pilocarpine Hydrochloride Ophthalmic Solution are determined by the severity of the elevated intraocular pressure and miotic response of the patient. To limit systemic exposure to pilocarpine, patients may be instructed to perform punctal occlusion for 2 minutes after instillation of Pilocarpine Hydrochloride Ophthalmic Solution.2.2 Management of Acute Angle-Closure Glaucoma
Prior to Pilocarpine Hydrochloride Ophthalmic Solution use, treatment with secretory suppressants and hyperosmotic agents may be needed to lower IOP below 50 mmHg and relieve iris ischemia.
For initial management of acute angle-closure glaucoma, one drop of Pilocarpine Hydrochloride Ophthalmic Solution 1% or 2% may be applied topically in the eye(s) up to three times over a 30-minute period.
If laser iridoplasty or iridomy is used to break the attack, one drop of Pilocarpine Hydrochloride Ophthalmic Solution 4% should be administered prior to the procedure. Following laser iridoplasty, one drop of Pilocarpine Hydrochloride Ophthalmic Solution 1% should be administered four times daily until an iridotomy can be performed.2.3 Prevention of Postoperative Elevated IOP Associated with Laser Surgery
One drop of Pilocarpine Hydrochloride Ophthalmic Solution 1%, 2% or 4% (or two drops administered five minutes apart) should be applied topically in the eye(s) 15 to 60 minutes prior to surgery.
2.4 Induction of Miosis
One drop of Pilocarpine Hydrochloride Ophthalmic Solution 1%, 2% or 4% (or two drops administered five minutes apart) should be applied topically in the eye(s).
2.5 Use with Other Topical Ophthalmic Medications
Pilocarpine may be used in combination with beta-blockers, carbonic anhydrase inhibitors, sympathomimetics or hyperosmotic agents. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart.
2.6 Use in Pediatric Patients
In children under 2 years of age, one drop of Pilocarpine Hydrochloride Ophthalmic Solution 1% should be applied topically in the eye(s) three times daily. Children 2 years of age and over should be dosed as for adults. For the induction of miosis prior to goniotomy or trabeculotomy in children, one drop of Pilocarpine Hydrochloride Ophthalmic Solution 1% or 2% should be applied topically in the eye 15 to 60 minutes prior to surgery.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Poor Illumination
Patients should be advised to exercise caution in night driving and other hazardous occupations in poor illumination. In addition, miotics may cause accommodative spasm. Patients should be advised not to drive or use machinery if vision is not clear.
5.2 Pre-existing Retinal Disease
As with all miotics, rare cases of retinal detachment have been reported when used in certain susceptible individuals and those with pre-existing retinal disease; therefore, a thorough examination of the retina including funduscopy is advised in all patients prior to the initiation of therapy.
5.3 Iritis
Pilocarpine Hydrochloride Ophthalmic Solution is not recommended to be used when iritis is present.
5.4 Primary Congenital Glaucoma
Caution is advised when using Pilocarpine Hydrochloride Ophthalmic Solution in pediatric patients with primary congenital glaucoma for control of intraocular pressure (IOP) as cases of a paradoxical increase in IOP have been reported. In addition, the use of Pilocarpine Hydrochloride Ophthalmic Solution is not recommended in pediatric patients diagnosed with glaucoma secondary to anterior segment dysgenesis or uveitis (especially if uveitis is active).
-
6 ADVERSE REACTIONS
Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described below reflect exposure in four controlled clinical trials of 90 days to 2 years duration in 317 patients diagnosed with open-angle glaucoma or ocular hypertension. In the four clinical trials, patients were treated with Pilocarpine Hydrochloride Ophthalmic Solution 2%, two to four times daily or with Pilocarpine Hydrochloride Ophthalmic Solution 1%, 1.75% or 2% in fixed combination with betaxolol 0.25%, two or three times daily. The most frequently reported adverse reactions occurring in ≥ 5% of patients in the pilocarpine 2% populations were: headache/browache, accommodative change, blurred vision, eye irritation, visual impairment (dim, dark, or “jumping” vision), and eye pain.
The adverse reaction profile reported for the use of Pilocarpine Hydrochloride Ophthalmic Solution in pediatric patients is comparable to that seen in adult patients. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy. Category C. Animal reproduction studies have not been conducted with pilocarpine hydrochloride. It is also not known whether pilocarpine hydrochloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Pilocarpine Hydrochloride Ophthalmic Solution should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Pilocarpine Hydrochloride Ophthalmic Solution is administered to a nursing woman.
-
10 OVERDOSAGE
Systemic toxicity following topical ocular administration of pilocarpine is rare, but occasionally patients who are sensitive may develop sweating and gastrointestinal overactivity following the suggested dosage and administration. Overdosage can produce sweating, salivation, nausea, tremors and slowing of the pulse and a decrease in blood pressure. In moderate overdosage, spontaneous recovery is to be expected and is aided by intravenous fluids to compensate for dehydration. For patients demonstrating severe poisoning, atropine, the pharmacologic antagonist to pilocarpine, should be used.
-
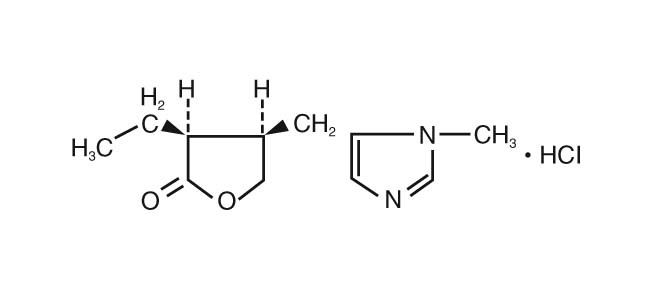
11 DESCRIPTION
Pilocarpine Hydrochloride Ophthalmic Solution is a cholinergic agonist prepared as a sterile topical ophthalmic solution. The active ingredient is represented by the chemical structure:
Established name: pilocarpine hydrochloride Chemical name: 2(3H)-furanone, 3-ethyldihydro-4-[(1-methyl-1H-imidazol-5-yl)-methyl]- monohydrochloride, (3S-cis)-. Molecular Formula: C11H16N2O2 HCl. Molecular Weight: 244.72.
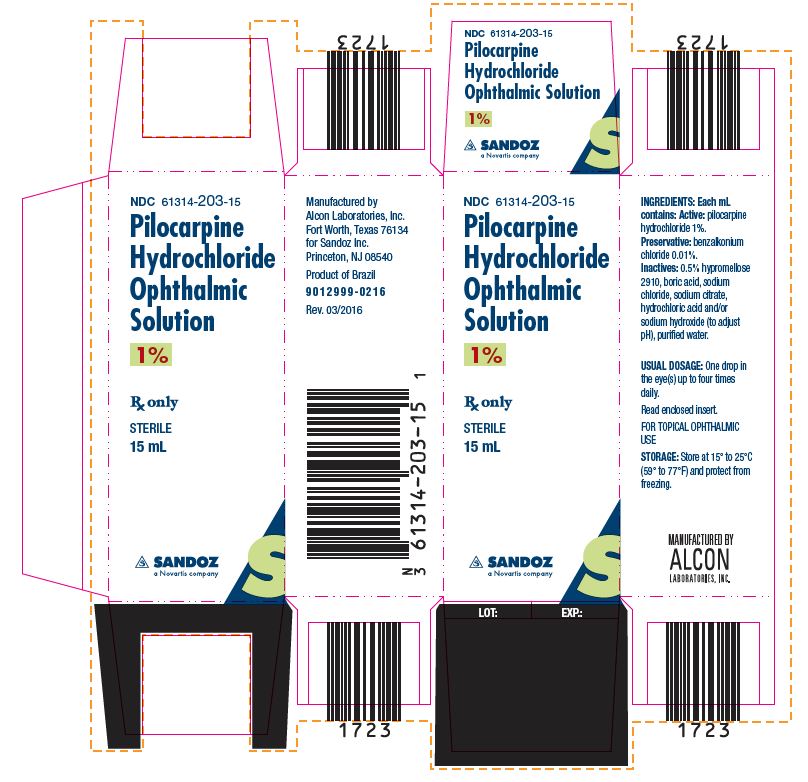
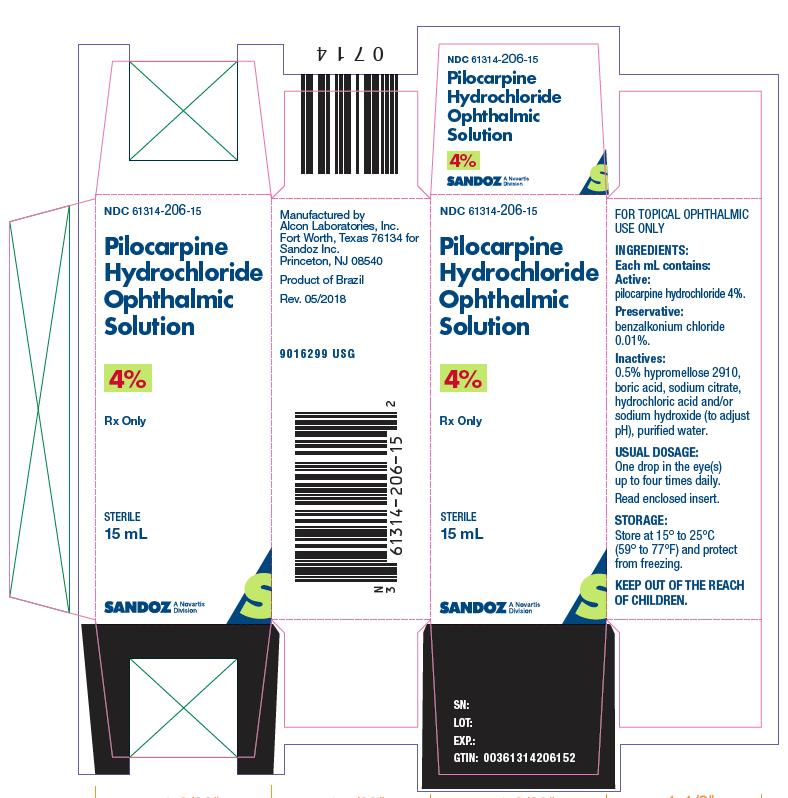
Each mL of Pilocarpine Hydrochloride Ophthalmic Solution contains: Active: pilocarpine hydrochloride 1% (10 mg/mL), 2% (20 mg/mL), or 4% (40 mg/mL). Preservative: benzalkonium chloride 0.01%. Inactives: hypromellose 2910, boric acid, sodium citrate, sodium chloride (present in 1% only); hydrochloric acid and/or sodium hydroxide (to adjust pH); purified water. Pilocarpine Hydrochloride Ophthalmic Solution has a pH of 3.5 to 5.5 and an osmolality of 290 to 350 mOsm/kg (1% and 2% products) and 550 to 600 mOsm/kg (4% product). -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pilocarpine hydrochloride is a direct acting cholinergic parasympathomimetic agent which acts through direct stimulation of muscarinic receptors and smooth muscle such as the iris and secretory glands. Pilocarpine contracts the ciliary muscle, causing increased tension on the scleral spur and opening of the trabecular meshwork spaces to facilitate outflow of aqueous humor. Outflow resistance is reduced, lowering intraocular pressure (IOP). Pilocarpine also produces miosis through contraction of the iris sphincter muscle. Miosis relieves appositional angle narrowing and closure, which lowers IOP in certain types of angle-closure glaucoma.
12.3 Pharmacokinetics
Systemic exposure to pilocarpine was evaluated in 14 healthy subjects administered 2 drops of pilocarpine hydrochloride ophthalmic solution 4% to both eyes four times daily for eight days. A comparison of Cmax values on Days 5 and 8 indicated that pilocarpine concentrations in plasma reached steady-state following topical administration of Pilocarpine Hydrochloride Ophthalmic Solution 4%. The mean (SD) Cmax and AUC0-last values on Day 8 were 3.7 (3.2) ng/mL and 7.7 (8.4) ng×hour/mL, respectively. The Tmax values on Day 8 ranged from 0.5 to 1 hour.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
In clinical trials reported in the medical literature, pilocarpine ophthalmic solution reduced intraocular pressure (IOP) by 3-7 mmHg in patients with open-angle glaucoma. Pilocarpine ophthalmic solution has also been shown to be effective in the induction of miosis, in the prevention of postoperative elevated IOP, and in the management of acute angle-closure glaucoma.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Pilocarpine Hydrochloride Ophthalmic Solution 1%, 2% and 4% is supplied sterile in natural low density polyethylene plastic ophthalmic DROP-TAINER®* dispensers and green low density polyethylene tips with green polypropylene caps.
15 mL in 15 mL bottles
1%: NDC 61314-203-15
2%: NDC 61314-204-15
4%: NDC 61314-206-15
STORAGE: Store at 15°- 25°C (59° - 77°F) and protect from freezing. -
17 PATIENT COUNSELING INFORMATION
17.1 Avoiding Contamination of the Product
Do not touch dropper tip to any surface, as this may contaminate the contents.
17.2 Night Driving
Caution is advised with night driving and when hazardous activities are undertaken in poor illumination.
17.3 Accommodative Spasm
Pilocarpine Hydrochloride Ophthalmic Solution may cause problems when changing focus between near objects and distant objects. Do not drive or use machinery if vision is not clear.
17.4 Contact Lens Wear
Contact lens should be removed prior to the instillation of Pilocarpine Hydrochloride Ophthalmic Solution. Wait 10 minutes after dosing before reinserting contact lenses.
17.5 Concomitant Topical Ocular Therapy
If more than one topical ophthalmic medication is being used, the medicines must be administered at least 5 minutes apart.
17.6 Systemic Exposure
To limit exposure to pilocarpine to the eye alone, close eyes gently and apply pressure with finger to the corner of eye by the nose for 2 minutes after instillation of Pilocarpine Hydrochloride Ophthalmic Solution.
Rx Only
Printed in USA
*DROP-TAINER is a registered trademark of Alcon Research, Ltd.
Manufactured by
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 for
Sandoz Inc.
Princeton, NJ 08540
9007060-1011 -
PRINCIPAL DISPLAY PANEL
NDC 61314-203-15
PilocarpineHydrochloride
Ophthalmic
Solution
1%
Rx only
STERILE
15 mL
SANDOZ
NDC 61314-203-15
Pilocarpine Hydrochloride Ophthalmic Solution
1%
Rx only
STERILE 15 mL
SANDOZ
NDC 61314-204-15
PilocarpineHydrochloride
Ophthalmic
Solution
2%
Rx only
STERILE
15 mL
SANDOZ
NDC 61314-206-15
PilocarpineHydrochloride
Ophthalmic
Solution
4%
Rx only
STERILE
15 mL
SANDOZ
-
INGREDIENTS AND APPEARANCE
PILOCARPINE HYDROCHLORIDE
pilocarpine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 61314-203 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Pilocarpine Hydrochloride (UNII: 0WW6D218XJ) (Pilocarpine - UNII:01MI4Q9DI3) Pilocarpine Hydrochloride 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) Hypromellose 2910 (15 Mpa.s) (UNII: 36SFW2JZ0W) Boric Acid (UNII: R57ZHV85D4) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) Sodium Chloride (UNII: 451W47IQ8X) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61314-203-15 1 in 1 CARTON 02/21/1996 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA200890 02/21/1996 PILOCARPINE HYDROCHLORIDE
pilocarpine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 61314-204 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Pilocarpine Hydrochloride (UNII: 0WW6D218XJ) (Pilocarpine - UNII:01MI4Q9DI3) Pilocarpine Hydrochloride 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) Hypromellose 2910 (15 Mpa.s) (UNII: 36SFW2JZ0W) Boric Acid (UNII: R57ZHV85D4) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61314-204-15 1 in 1 CARTON 02/21/1996 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA200890 02/21/1996 PILOCARPINE HYDROCHLORIDE
pilocarpine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 61314-206 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Pilocarpine Hydrochloride (UNII: 0WW6D218XJ) (Pilocarpine - UNII:01MI4Q9DI3) Pilocarpine Hydrochloride 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) Hypromellose 2910 (15 Mpa.s) (UNII: 36SFW2JZ0W) Boric Acid (UNII: R57ZHV85D4) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61314-206-15 1 in 1 CARTON 02/21/1996 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA200890 02/21/1996 Labeler - Sandoz Inc (005387188)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.