Lidocaine and Menthol Patch
Lidocaine and Menthol by
Drug Labeling and Warnings
Lidocaine and Menthol by is a Otc medication manufactured, distributed, or labeled by 7T Pharma LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LIDOCAINE AND MENTHOL- lidocaine and menthol patch
7T Pharma LLC
----------
Lidocaine and Menthol Patch
Lidocaine 4% and Menthol 4% Patch
7T Pharma, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Lidocaine 4% and Menthol 4% Patch
Drug Facts
Purpose
Lidocaine 4%..................................................................................................Topical Anesthetic
Menthol 4%.....................................................................................................Topical Analgesic
Uses
Temporarily relieves minor aches and muscle pains associated with:
arthritis
simple backache
strains
muscle soreness
muscle stiffness
Warnings
For external use only.
Read all warnings and directions before use.
Do not use
More than one patch at a time.
On wounds, cuts, damaged or infected skin as well as in the eyes, mouth, genitals, or any other mucous membranes.
With a heating pad or cover with bandage.
If you are allergic to any ingredients of this product
Allergy Alert: if you are allergic to any inactive ingredient of this product, contact a doctor before use.
When using this product
Use only as directed.
Avoid contact with eyes, mucous membranes, or rashes
Do not tightly wrap or bandage the treated area
Stop use and ask a physician:
If pregnant or breast feeding
If localized skin reactions occur, such as rash, itching, redness, irritation, pain, swelling, and blistering
If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a physician.
Directions
Adults and children 12 years of age and older:
Apply 1 patch to the affected area of intact skin 1 to 2 times daily or as directed. Do not leave patch on for more than 8 hours at a time.
- Clean and dry the affected area.
- Open pouch and remove one patch.
- Remove any protective film and apply directly to affected area of pain. Apply immediately after removal from the protective envelope.
- Wash hands with soap and water after handling the patches.
- Reseal pouch containing unused patches after each use. Do not store patch outside the sealed envelope.
- Fold used patches so that the adhesive side sticks to itself and safely discard used patches or pieces of cut patches where children and pets cannot get to them.
Children under 12 years: Ask a physician
Other information
Store at room temperature 15°-30°C (59°-86°F)
Avoid storing product in direct sunlight and protect product from excessive moisture.
Inactive ingredients
Aqua (Deionized Water), Glycerine, Sodium Polyacrylate, Polysorbate 80, EDTA Disodium salt, Methylparaben, Citrus Medica Limonum (Lemon) Peel Oil, Aloe Barbadesis Leaf (Aloe Vera Gel) Juice, Diazolidinyl Urea, Iodopropynyl Butylcarbamate, Propylparaben
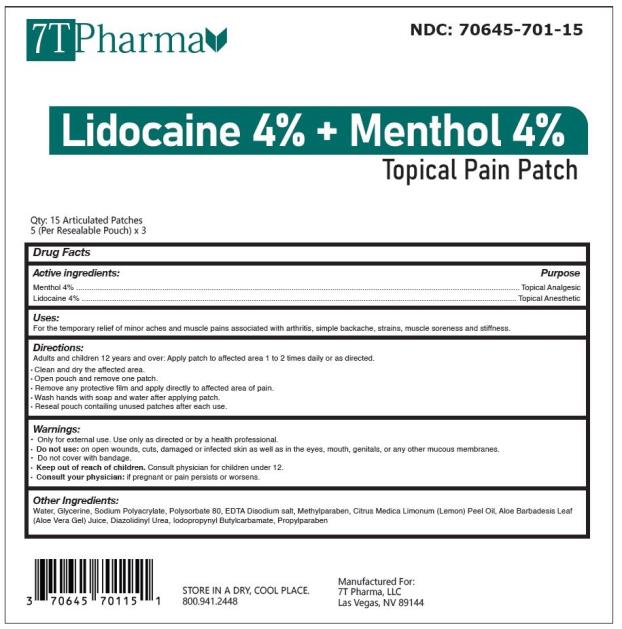
PRINCIPAL DISPLAY PANEL
Lidocaine 4% and Menthol 4% Topical Pain Patch
NDC: 70645-701-15
15 Articulated Patches (5 per Resealable Pouch X 3 )per Box
Manufactured for:
7T Pharma, LLC
Las Vegas, NV 89144
| LIDOCAINE AND MENTHOL
lidocaine and menthol patch |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - 7T Pharma LLC (080220022) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.