FIRSTCARE CHILDRENS ALLERGY RELIEF- diphenhydramine hcl bar, chewable
FIRSTCARE CHILDRENS ALLERGY RELIEF by
Drug Labeling and Warnings
FIRSTCARE CHILDRENS ALLERGY RELIEF by is a Otc medication manufactured, distributed, or labeled by USpharma Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
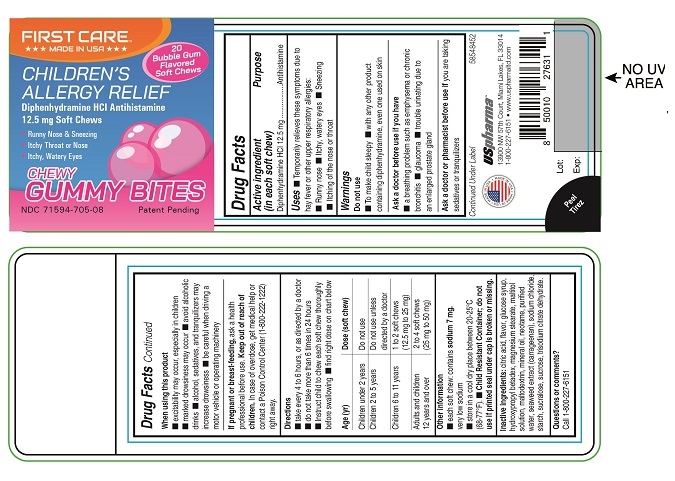
- Active ingredient (in each soft chew)
- Purpose
- Uses
-
Warnings
Do not use
- To make child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
-
Directions
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 times in 24 hours
- Instruct child to chew each soft chew thoroughly before swallowing
- find right dose on chart below
Age (yr)
Dose (soft chew)
Children under 2 years
Do not use
Children 2 to 5 years
Do not use unless directed by a doctor
Children 6 to 11 years
1 to 2 soft chews (12.5 mg to 25 mg)
Adults and children 12 years and over
2 to 4 soft chews (25 mg to 50 mg)
- SPL UNCLASSIFIED SECTION
- Inactive ingredients:
- SPL UNCLASSIFIED SECTION
-
Package/Label Principal Display Panel
FIRSTCARETM
***MADE IN USA***
20 Bubble Gum Flavored Soft Chews
CHILDEREN'S ALLERGY RELIEF
Diphenhydramine HCl Anthihistamine 12.5 mg Soft Chews
Runny Nose & Sneezing
Itchy throat or Nose
Itchy, Water Eyes
CHEWY GUMMY BITESNDC: 71594-705-08 Patent Pending
-
INGREDIENTS AND APPEARANCE
FIRSTCARE CHILDRENS ALLERGY RELIEF
diphenhydramine hcl bar, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71594-705 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg Inactive Ingredients Ingredient Name Strength TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SODIUM CHLORIDE (UNII: 451W47IQ8X) SUCRALOSE (UNII: 96K6UQ3ZD4) NEOTAME (UNII: VJ597D52EX) CORN SYRUP (UNII: 9G5L16BK6N) CARRAGEENAN (UNII: 5C69YCD2YJ) MALTITOL (UNII: D65DG142WK) HYDROXYPROPYL BETADEX (UNII: 1I96OHX6EK) MALTODEXTRIN (UNII: 7CVR7L4A2D) SUCROSE (UNII: C151H8M554) MINERAL OIL (UNII: T5L8T28FGP) STARCH, CORN (UNII: O8232NY3SJ) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color yellow (Light yellow to golden brown) Score no score Shape RECTANGLE Size 18mm Flavor BUBBLE GUM Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71594-705-08 20 in 1 BOTTLE; Type 0: Not a Combination Product 05/21/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 05/21/2020 Labeler - USpharma Ltd (080664601) Registrant - USpharma Ltd (080664601) Establishment Name Address ID/FEI Business Operations USpharma Ltd 080664601 manufacture(71594-705) , pack(71594-705)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.