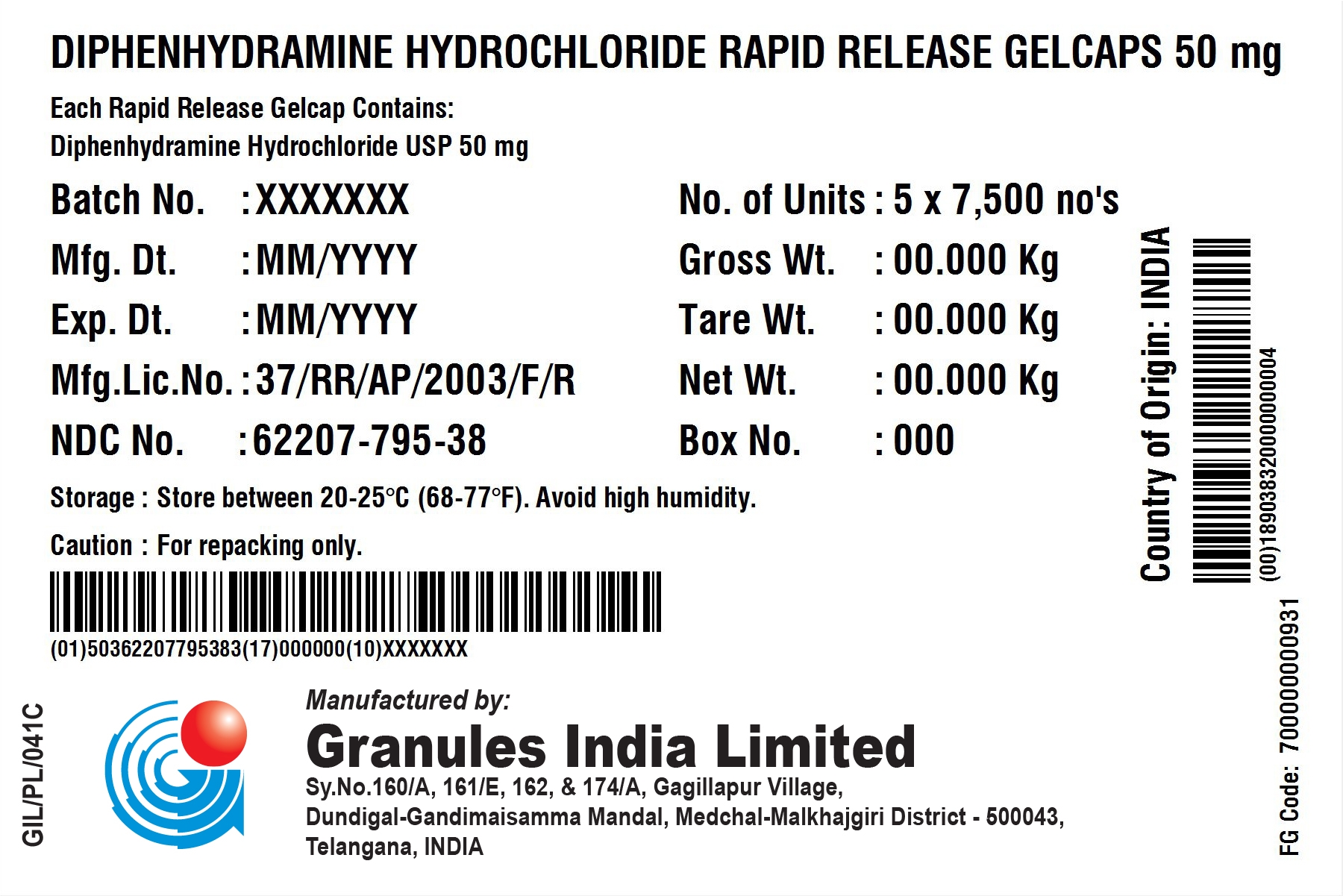
Diphenhydramine Hydrochloride Rapid Release Gelcaps 50 mg
Diphenhydramine Hydrochloride by
Drug Labeling and Warnings
Diphenhydramine Hydrochloride by is a Other medication manufactured, distributed, or labeled by Granules India Limited, Granules India limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DIPHENHYDRAMINE HYDROCHLORIDE- diphenhydramine hydrochloride capsule
Granules India Limited
----------
Diphenhydramine Hydrochloride Rapid Release Gelcaps 50 mg
| DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride capsule |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Granules India Limited (915000087) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Granules India limited | 918609236 | manufacture(62207-795) | |
Revised: 7/2023
Document Id: 01c1da06-dc5e-32c3-e063-6294a90a2a17
Set id: 54c1d306-dc2e-2885-e054-00144ff8d46c
Version: 4
Effective Time: 20230731
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.