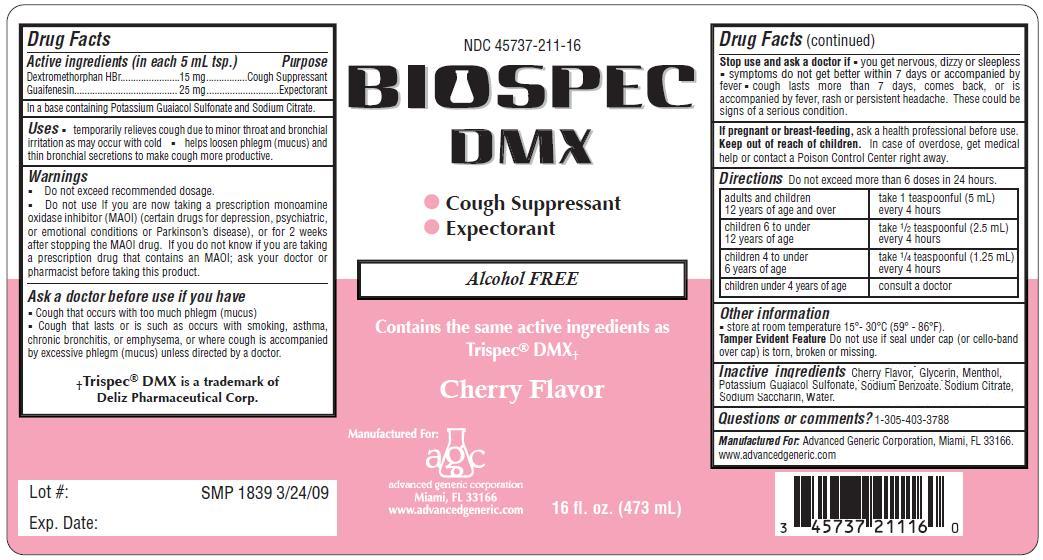
BIOSPEC DMX- dextromethorphan, guaifenesin liquid
Biospec by
Drug Labeling and Warnings
Biospec by is a Otc medication manufactured, distributed, or labeled by Advanced Generic Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings
- Do not exceed recommended dosage.
- Do not use If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if you are taking a prescription drug that contains an MAOI; ask your doctor or pharmacist before taking this product.
- Cough that occurs with too much phlegm (mucus)
- Cough that lasts or is such as occurs with smoking, asthma, chronic bronchitis, or emphysema, or where cough is accompanied by excessive phlegm (mucus) unless directed by a doctor.
- DO NOT USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions Do not take more than 6 doses in any 24-hour period, unless directed by a physician
adults and children 12 years and over, 1 teaspoonfuls (5 ml) every 4 hours
children 6 years to under 12 years, 1/2 teaspoonful (2.5 ml) every 4 hours
children 4 to 6 years, 1/4 teaspoonful (1.25 ml) every 4 hours
children under 4 years of age, consult a doctor
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BIOSPEC DMX
dextromethorphan, guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 45737-211 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dextromethorphan Hydrobromide (UNII: 9D2RTI9KYH) (Dextromethorphan - UNII:7355X3ROTS) Dextromethorphan Hydrobromide 15 mg in 5 mL Guaifenesin (UNII: 495W7451VQ) (Guaifenesin - UNII:495W7451VQ) Guaifenesin 25 mg in 5 mL Inactive Ingredients Ingredient Name Strength Menthol (UNII: L7T10EIP3A) Water (UNII: 059QF0KO0R) POTASSIUM GUAIACOLSULFONATE (UNII: TTK33Z47F1) Glycerin (UNII: PDC6A3C0OX) SODIUM BENZOATE (UNII: OJ245FE5EU) Sodium Citrate (UNII: 1Q73Q2JULR) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Product Characteristics Color Score Shape Size Flavor CHERRY (Cherry Flavor) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 45737-211-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 10/01/2009 Labeler - Advanced Generic Corporation (831762971)
Trademark Results [Biospec]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BIOSPEC 79299577 not registered Live/Pending |
Camlin Limited 2020-11-06 |
 BIOSPEC 75241684 2156493 Live/Registered |
Mannington Mills, Inc. 1997-02-13 |
 BIOSPEC 75064185 not registered Dead/Abandoned |
Hutchinson Technology Incorporated 1996-02-23 |
 BIOSPEC 74676447 not registered Dead/Abandoned |
Shimadzu Corporation 1995-05-18 |
 BIOSPEC 74635308 2049648 Dead/Cancelled |
Naturade Products, Inc. 1995-02-17 |
 BIOSPEC 73486435 1404648 Live/Registered |
BRUKER ANALYTISCHE MESSTECHNIK GMBH 1984-06-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.