SIGNIFOR LAR- pasireotide kit
Signifor LAR by
Drug Labeling and Warnings
Signifor LAR by is a Prescription medication manufactured, distributed, or labeled by Novartis Pharmaceuticals Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SIGNIFOR LAR safely and effectively. See full prescribing information for SIGNIFOR LAR.
SIGNIFOR® LAR (pasireotide) for injectable suspension, for intramuscular use
Initial U.S. Approval: 2012
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Evaluate fasting plasma glucose, hemoglobin A1c (HbA1c), liver enzyme tests, electrocardiogram (ECG), serum magnesium, and serum potassium prior to starting. (2.1)
- Optimize glucose control in patients with poorly controlled diabetes mellitus prior to starting. (2.1)
- Must be administered by a health care professional only by intramuscular injection in the right or left gluteus immediately after reconstitution. (2.2)
- Initial Dose:
- Adjust dose based on response and tolerability. (2.4)
- Patients with Hepatic Impairment:
- Follow reconstitution and administration instructions. (2.6)
DOSAGE FORMS AND STRENGTHS
SIGNIFOR LAR for injectable suspension: 10 mg, 20 mg, 30 mg, 40 mg, and 60 mg, powder in a vial to be reconstituted with the provided 2 mL diluent. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Hyperglycemia and Diabetes: Sometimes severe. Monitor glucose levels periodically during therapy. Monitor glucose levels more frequently in the months that follow initiation or discontinuation of SIGNIFOR LAR therapy and following SIGNIFOR LAR dose adjustment. Use anti-diabetic treatment if indicated per standard of care. (2.1, 5.1)
- Bradycardia and QT Prolongation: Use with caution in at-risk patients; Evaluate ECG and electrolytes prior to dosing and periodically while on treatment. (2.1, 5.2, 7.1)
- Liver Test Elevations: Evaluate liver enzyme tests prior to and during treatment. (2.1, 5.3)
- Cholelithiasis and Complications of Cholelithiasis: Monitor periodically. Discontinue if complications of cholelithiasis are suspected (5.4)
- Pituitary Hormone Deficiency(ies): Monitor for occurrence periodically and treat if clinically indicated. (5.5)
ADVERSE REACTIONS
Adverse drug reactions associated with SIGNIFOR LAR and occurring in ≥ 20% of patients were diarrhea, cholelithiasis, hyperglycemia and diabetes mellitus. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Females and Males of Reproductive Potential: Advise premenopausal females of the potential for an unintended pregnancy (8.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Acromegaly
1.2 Cushing’s Disease
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Baseline Evaluations Prior to Initiation of SIGNIFOR LAR
2.2 Important Administration Instructions
2.3 Recommended Initial Dose
2.4 Dose Adjustment and Monitoring
2.5 Dose in Patients With Hepatic Impairment
2.6 Reconstitution and Intramuscular Injection Instructions
2.7 Missed Dose
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hyperglycemia and Diabetes
5.2 Bradycardia and QT Prolongation
5.3 Liver Test Elevations
5.4 Cholelithiasis and Complications of Cholelithiasis
5.5 Pituitary Hormone Deficiency(ies)
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on SIGNIFOR LAR
7.2 Effect of SIGNIFOR LAR on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Drug-Naïve Patients with Acromegaly
14.2 Patients with Acromegaly Inadequately Controlled on Other Somatostatin Analogs
14.3 Patients with Cushing’s Disease
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Baseline Evaluations Prior to Initiation of SIGNIFOR LAR
Prior to the initiation of SIGNIFOR LAR, it is recommended that patients have the following baseline evaluations:
- Fasting plasma glucose (FPG) and hemoglobin A1c (HbA1c) [see Warnings and Precautions (5.1)]
- Liver tests [see Warnings and Precautions (5.3)]
- Electrocardiogram (ECG), serum potassium and serum magnesium levels [see Warnings and Precautions (5.2)]
Patients with poorly controlled diabetes mellitus, who have inadequate glucose control, should have anti-diabetic therapy optimized prior to starting SIGNIFOR LAR [see Warnings and Precautions (5.1)].
2.2 Important Administration Instructions
SIGNIFOR LAR must be reconstituted by a trained healthcare professional immediately before use. Illustrations on reconstitution are found in Instructions for Use [see Dosage and Administration (2.6)].
SIGNIFOR LAR must be inspected visually before use. The suspension should appear free of foreign particulates and should be homogeneous after mixing.
SIGNIFOR LAR must be administered by a trained healthcare professional only by intramuscular injection in the right or left gluteus immediately after reconstitution. SIGNIFOR LAR must never be administered intravenously.
2.3 Recommended Initial Dose
Acromegaly
The recommended initial dose of SIGNIFOR LAR for the treatment of acromegaly is 40 mg administered by intramuscular injection once every 4 weeks (every 28 days) [see Dosage and Administration (2.6)].
Cushing’s Disease
The recommended initial dose of SIGNIFOR LAR for the treatment of Cushing’s disease is 10 mg administered by intramuscular injection once every 4 weeks (every 28 days) [see Dosage and Administration (2.6)].
2.4 Dose Adjustment and Monitoring
Acromegaly
The dose may be increased to a maximum of 60 mg for patients who have not normalized growth hormone (GH) and/or age and sex adjusted insulin-like growth factor-1 (IGF-1) levels after 3 months of treatment with SIGNIFOR LAR at 40 mg and who tolerate this dose.
Management of SIGNIFOR LAR-related adverse reactions or over-response to treatment (age and sex adjusted IGF-1 less than the lower limit of normal) may require dose reduction. The dose may be decreased, either temporarily or permanently, by 20 mg decrements [see Warnings and Precautions (5)].
Cushing’s Disease
Following 4 months of treatment with the initial dose of 10 mg once every 28 days, the dose may be increased for patients who have not normalized 24-hour urinary free cortisol (UFC) and who tolerate this dose, up to a maximum dose of 40 mg once every 28 days.
Management of suspected adverse reactions or over-response to treatment (e.g., cortisol levels less than the lower limit of the normal range or in the low part of the normal range in patients with symptoms suggestive of adrenal insufficiency) may require dose reduction to the previous tolerated dose, dose interruption, or drug discontinuation of SIGNIFOR LAR. For patients treated with 10 mg once every 28 days, the dose may be either interrupted or discontinued [see Warnings and Precautions (5)].
2.5 Dose in Patients With Hepatic Impairment
For patients with moderately impaired hepatic function (Child-Pugh B) [see Use in Specific Populations (8.6)]:
- Acromegaly: The recommended initial dose for acromegaly patients with moderately impaired hepatic function is 20 mg once every 4 weeks and the maximum recommended dose is 40 mg once every 4 weeks.
- Cushing’s Disease: The recommended initial dose for Cushing’s disease patients with moderately impaired hepatic function is 10 mg once every 4 weeks and the maximum recommended dose is 20 mg once every 4 weeks.
Avoid use in patients with severe hepatic impairment (Child-Pugh C) [see Use in Specific Populations (8.6)].
2.6 Reconstitution and Intramuscular Injection Instructions
After reconstitution of the SIGNIFOR LAR vial with the provided 2 mL diluent, the intramuscular injectable suspension will have a final concentration of:
Strength per Vial Final Concentration When Reconstituted
(total product strength per total volume)Final Concentration When
Reconstituted (per mL)10 mg 10 mg/2 mL 5 mg/mL 20 mg 20 mg/2 mL 10 mg/mL 30 mg 30 mg/2 mL 15 mg/mL 40 mg 40 mg/2 mL 20 mg/mL 60 mg 60 mg/2 mL 30 mg/mL The entire contents of the reconstituted solution should be administered immediately.
PAY PARTICULAR ATTENTION:
There are 2 critical steps in the reconstitution of SIGNIFOR LAR. Not following these 2 steps could result in failure to deliver the drug appropriately.
1) The injection kit must reach room temperature (see Step 1 in Instructions for Use). Remove the SIGNIFOR LAR injection kit from refrigerated storage and let the kit stand at room temperature for a minimum of 30 minutes before reconstitution, but do not exceed 24 hours.
2) After adding the diluent solution, shake the vial moderately in a horizontal direction for a minimum of 30 seconds until uniform suspension is formed (see Step 4 in Instructions for Use).
The following items are included in the injection kit:
a) One vial containing SIGNIFOR LAR powder
b) One prefilled syringe containing the diluent solution for reconstitution
c) One vial adapter for drug product reconstitution
d) One safety injection needle (20G x 1.5”)
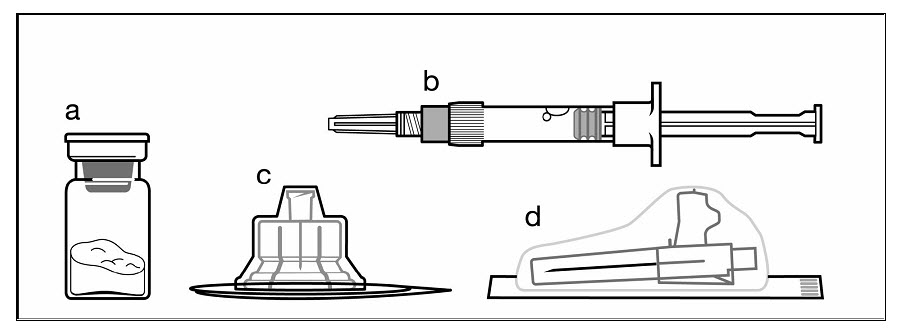
Figure 1. Items Included in Injection Kit
SIGNIFOR LAR suspension must only be reconstituted immediately before administration.
Follow the directions in the Instructions for Use to ensure proper reconstitution of SIGNIFOR LAR before intramuscular injection.
SIGNIFOR LAR should only be administered by a trained healthcare professional.
Instructions for Use
Step 1
Remove the SIGNIFOR LAR for injectable suspension kit from refrigerated storage.
PAY PARTICULAR ATTENTION: It is essential to start the reconstitution process only after the injection kit has reached room temperature. Let the kit stand at room temperature for at least 30 minutes before starting reconstitution, but not more than 24 hours.
Note: The kit can be re-refrigerated if needed.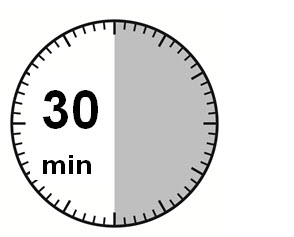
Step 2
Remove the plastic cap from the vial and clean the rubber stopper with an alcohol wipe.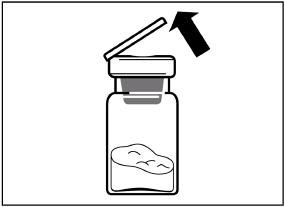
Remove the lid film of the vial adapter packaging, but do NOT remove the vial adapter from its packaging.
Holding the vial adapter packaging, position the vial adapter on top of the vial and push it fully down so that it snaps in place. You will hear an audible “click” when the vial adapter snaps in place.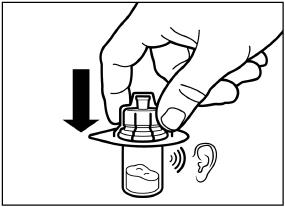
Lift the packaging off the vial adapter with a vertical movement. 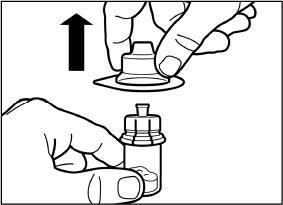
Step 3
Remove the cap from the syringe prefilled with diluent solution and screw the syringe onto the vial adapter.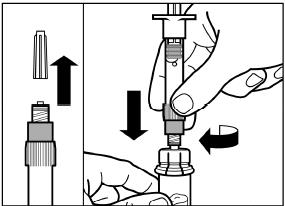
Slowly push the plunger all the way down to transfer all the diluent solution in the vial. 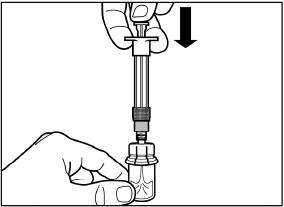
Step 4
ATTENTION: Keep the plunger pressed and shake the vial moderately in a horizontal direction for a minimum of 30 seconds so that the powder is completely suspended. Repeat moderate shaking for another 30 seconds if the powder is not completely suspended.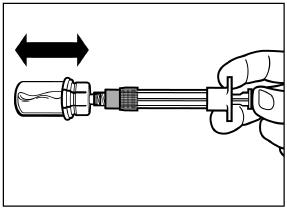
Step 5
Turn the syringe and vial upside down, slowly pull the plunger back and draw the entire content from the vial into the syringe.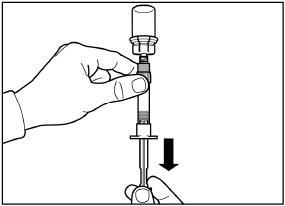
Unscrew the syringe from the vial adapter. 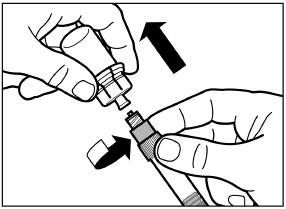
Step 6
Screw the safety injection needle onto the syringe.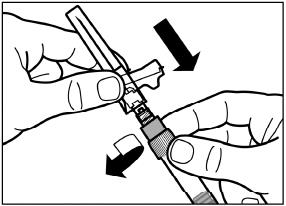
Pull the protective cover straight off the needle.
To avoid sedimentation and maintain a uniform suspension, you may gently shake the syringe.
Gently tap the syringe to remove any visible bubbles and expel them from the syringe.
The reconstituted SIGNIFOR LAR is now ready for immediate administration.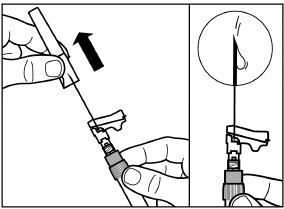
Step 7
SIGNIFOR LAR must only be given by intramuscular injection and NEVER intravenously.
Prepare the injection site by wiping with an alcohol wipe.
Insert the needle fully into the left or right gluteus at a 90° angle to the skin.
Slowly pull back the plunger to check that no blood vessel has been penetrated (reposition if a blood vessel has been penetrated).
Slowly depress the plunger until the syringe is empty. Withdraw the needle from the injection site and activate the safety guard (as shown in Step 8).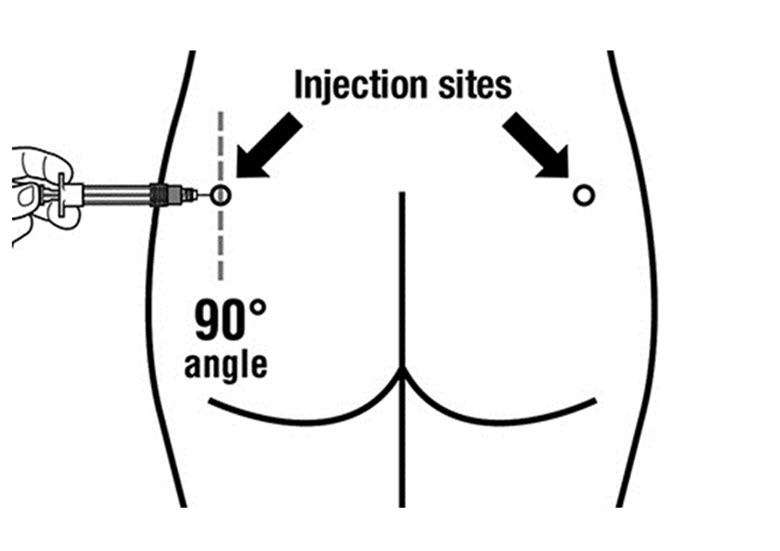
Step 8
Activate the safety guard over the needle using 1 of the 2 methods shown:
either press the hinged section of the safety guard down onto a hard surface (Figure A),
or push the hinge forward with your finger (Figure B).
An audible “click” will confirm proper activation of the safety guard.
Dispose of syringe immediately in a sharps container.
Any unused product or waste material should be disposed of in accordance with local requirements.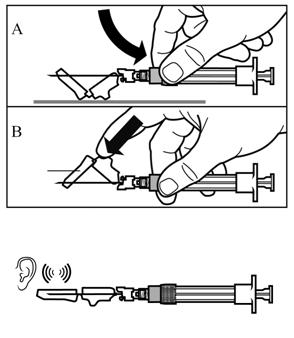
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hyperglycemia and Diabetes
SIGNIFOR LAR can cause increases in blood glucose levels which are sometimes severe. In the study of patients with acromegaly, 5 patients naïve to drug therapy exposed to SIGNIFOR LAR (2 of whom were normoglycemic at baseline) were hospitalized for blood glucose in the range of 359-506 mg/dL and none in the active comparator group. Two additional patients who had received active comparator in the main trial and were switched to SIGNIFOR LAR in the extension trial, were hospitalized for elevated glucose levels while on SIGNIFOR LAR treatment during the extension; one of those patients developed diabetic ketoacidosis. In the Cushing’s disease study, 2 patients were hospitalized for elevated blood glucose. Patients with poor baseline glycemic control are at higher risk of developing severe hyperglycemia [see Dosage and Administration (2.1)].
In clinical studies for acromegaly and Cushing’s disease, treatment with SIGNIFOR LAR caused an increase in the incidence of diabetes and prediabetes. A majority of patients, including those with normal glucose tolerance, prediabetes and diabetes, experienced increased glucose levels within the first 3 months of treatment with SIGNIFOR LAR [see Adverse Reactions (6.1)]. In the drug-naïve acromegaly study, the prevalence of diabetes increased from 30% at baseline to 60% at Month 12. In the study evaluating acromegaly patients previously treated with somatostatin analogs, the prevalence of diabetes increased from 71% at baseline to 87% at Month 6 in patients treated with SIGNIFOR LAR 40 mg, and from 60% to 84% in patients treated with SIGNIFOR LAR 60 mg. In the Cushing’s disease study, the prevalence of diabetes increased from 40% at baseline to 56% at Month 12.
Fasting plasma glucose and HbA1c should be assessed prior to starting treatment with SIGNIFOR LAR. In patients with poorly controlled diabetes mellitus, anti-diabetic treatment should be optimized per standard of care before SIGNIFOR LAR treatment is started. Blood glucose monitoring should be done weekly for the first 3 months after initiating SIGNIFOR LAR and the first 4- to 6 weeks after dose increases. Periodic monitoring should continue thereafter, as clinically appropriate.
Patients who develop significant hyperglycemia on SIGNIFOR LAR may require initiation of anti-diabetic therapy or adjustment in their current anti-diabetic therapy per standard of care. The optimal treatment for the management of SIGNIFOR LAR-induced hyperglycemia is not known. If hyperglycemia cannot be controlled despite medical management, the dose of SIGNIFOR LAR should be reduced or SIGNIFOR LAR should be discontinued.
After treatment discontinuation, fasting plasma glucose and hemoglobin A1c should be assessed if indicated. Patients on anti-diabetic therapy discontinuing SIGNIFOR LAR may require more frequent blood glucose monitoring and anti-diabetic drug therapy dose adjustment to mitigate the risk of hypoglycemia.
5.2 Bradycardia and QT Prolongation
Bradycardia
Bradycardia has been reported with the use of SIGNIFOR LAR [see Adverse Reactions (6.1)]. Patients with cardiac disease and/or risk factors for bradycardia, such as history of clinically significant bradycardia, high-grade heart block, or concomitant use of drugs associated with bradycardia, should be monitored. Adjustments in the dose of drugs known to slow the heart rate (e.g., beta-blockers, calcium channel blockers) and correction of electrolyte disturbances may be necessary when initiating or during the course of SIGNIFOR LAR treatment.
QT Prolongation
In cardiac electrophysiology studies (i.e., thorough QT studies) with pasireotide via subcutaneous route, QT prolongation occurred at therapeutic and supra-therapeutic doses [see Clinical Pharmacology (12.2)].
In the clinical studies, a corrected QT interval (i.e., QTcF) of greater than 480 ms was reported in 6 patients and an increase in the QTcF from baseline of greater than 60 ms was reported for seven patients on SIGNIFOR LAR. No patient on SIGNIFOR LAR had a QTcF value of greater than 500 ms [see Adverse Reactions (6.1), Clinical Pharmacology (12.2)].
SIGNIFOR LAR should be used with caution in patients who are at significant risk of developing prolongation of the QT interval, such as those listed below:
- with congenital long QT prolongation
- with uncontrolled or significant cardiac disease including recent myocardial infarction, congestive heart failure, unstable angina or clinically significant bradycardia
- on anti-arrhythmic therapy or other substances that are known to lead to QT prolongation
- with hypokalemia and/or hypomagnesemia
A baseline ECG is recommended prior to initiating therapy with SIGNIFOR LAR. Monitoring for an effect on the QT interval at the time of maximum drug concentration (21 days after injection) should be obtained in patients at risk. Hypokalemia or hypomagnesemia must be corrected prior to initiating SIGNIFOR LAR and should be monitored periodically during therapy.
5.3 Liver Test Elevations
Increases in liver enzymes have been observed with SIGNIFOR LAR. In all Phase 3 acromegaly studies and across all doses, alanine aminostranferase (ALT) or aspartate aminotransferase (AST) elevations greater than 3 times the upper limit of normal (ULN) were observed in 4% of acromegaly patients and ALT or AST elevations greater than 5 times the ULN were observed in 1% of acromegaly patients treated with SIGNIFOR LAR.
In the Phase 3 Cushing’s disease study and across all doses, ALT or AST elevations greater than 3 times the ULN were observed in 14% of Cushing’s disease patients and ALT or AST elevations greater than 5 times the ULN were observed in 5% of Cushing’s disease patients treated with SIGNIFOR LAR.
Assessment of liver function is recommended prior to treatment with SIGNIFOR LAR , and after the first 2- to 3 weeks, then monthly for 3 months. Thereafter, liver function should be monitored as clinically indicated. Patients who develop increased transaminase levels, should be monitored until values return to pre-treatment levels. Treatment with SIGNIFOR LAR should be discontinued if signs or symptoms suggestive of clinically significant liver impairment develop. Following discontinuation of treatment with SIGNIFOR LAR, patients should be monitored until resolution. Treatment should not be restarted, if the liver function abnormalities are suspected to be related to SIGNIFOR LAR.
5.4 Cholelithiasis and Complications of Cholelithiasis
Cholelithiasis was reported in 33% of drug-naïve and 10% of inadequately controlled (40 mg dose) acromegaly patients treated with SIGNIFOR LAR in clinical trials [see Adverse Reactions (6)]. Cholelithiasis was reported in 33% of Cushing’s disease patients treated with SIGNIFOR LAR. There have been postmarketing reports of cholelithiasis (gallstones) resulting in complications, including cholecystitis or cholangitis and requiring cholecystectomy in patients taking SIGNIFOR LAR. Patients should be monitored periodically. If complications of cholelithiasis are suspected, discontinue SIGNIFOR LAR and treat appropriately.
5.5 Pituitary Hormone Deficiency(ies)
Suppression of anterior pituitary hormones may occur on SIGNIFOR LAR. Monitoring pituitary function (e.g., thyroid, adrenal, gonadal) prior to initiation of therapy with SIGNIFOR LAR, as well as periodically during treatment, as clinically appropriate, is recommended. Patients should be monitored for and instructed on the signs and symptoms of adrenal insufficiency during therapy. If adrenal insufficiency is suspected, it should be confirmed and treated per standard of care with exogenous glucocorticoids at replacement doses.
-
6 ADVERSE REACTIONS
Clinically significant adverse reactions that appear in other sections of the labeling include:
- Hyperglycemia and Diabetes [see Warnings and Precautions (5.1)]
- Bradycardia and QT Prolongation [see Warnings and Precautions (5.2)]
- Liver Test Elevations [see Warnings and Precautions (5.3)]
- Cholelithiasis and Complications of Cholelithiasis [see Warnings and Precautions (5.4)]
- Pituitary Hormone Deficiency(ies) [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
Drug-Naïve Patients With Acromegaly
The data described in Table 1 are derived from an active-controlled trial in patients with acromegaly naïve to previous drug therapy [see Clinical Studies (14.1)]. The data reflect exposure of 178 patients with acromegaly to SIGNIFOR LAR for a mean duration of 43 weeks. In the overall study population, 52% were female and the average age of patients was 45 years.
Table 1 presents common adverse reactions associated with SIGNIFOR LAR in this study. These adverse reactions were not present at baseline or, if present, worsened from baseline and occurred in at least 5% of patients treated with SIGNIFOR LAR.
Table 1 – Adverse Reactions Occurring in Greater Than or Equal to 5% of Patients Exposed to SIGNIFOR LAR in Patients With Acromegaly Naïve to Drug Therapy *Diabetes mellitus includes the following PTs: diabetes mellitus and type 2 diabetes mellitus.
**Sinus bradycardia includes the following PTs: bradycardia and sinus bradycardia.
***Injection-site reaction related AEs includes the following PTs: injection-site pain, injection-site reaction, injection-site haematoma, injection-site pruritus, injection-site swelling, injection-site erythema.Adverse Reaction Type SIGNIFOR LAR
(40-60 mg)
%
N = 178Active Comparator
%
N = 180Hyperglycemia Related Adverse Reactions Hyperglycemia 29 8 Diabetes mellitus* 26 4 Blood glucose increased 8 2 Glycosylated hemoglobin increased 6 2 Hypoglycemia 5 7 Gastrointestinal Related Adverse Reactions Diarrhea 39 45 Abdominal pain 18 22 Nausea 14 22 Abdominal distension 12 12 Vomiting 8 7 Abdominal pain upper 6 8 Hepatobiliary Related Adverse Reactions Cholelithiasis 26 36 Cardiac Related Adverse Reactions Sinus bradycardia** 10 7 Hypertension 8 7 Nervous System Related Adverse Reactions Headache 19 26 Dizziness 10 11 Skin Related Adverse Reactions Alopecia 18 19 Infections Related Adverse Reactions Nasopharyngitis 16 16 Influenza 8 4 Upper respiratory tract infection 7 3 Cough 5 8 Laboratory Related Adverse Reactions Blood creatine phosphokinase increased 13 12 Alanine aminotransferase increased 8 4 Aspartate aminotransferase increased 6 4 Lipase increased 6 7 Weight decreased 5 4 General and Injection-Site Related Adverse Reactions Fatigue 10 10 Injection-site reaction*** 7 7 Musculoskeletal and Connective Tissue Related Adverse Reactions Arthralgia 10 12 Back pain 8 11 Pain in extremity 7 4 Blood Related Adverse Reactions Anemia 6 6 Other notable adverse reactions which occurred with a frequency of 5% or less for SIGNIFOR LAR were: adrenal insufficiency (3%); glucose tolerance impaired (1%); QT-prolongation (4%); blood amylase increased (2%).
Patients With Acromegaly Inadequately Controlled on Other Somatostatin Analogs at Baseline
The data described in Table 2 are derived from an active-controlled study in patients with acromegaly inadequately controlled at baseline on other somatostatin analogs [see Clinical Studies (14.2)]. These data reflect exposure of 63 and 62 patients to SIGNIFOR LAR 40 mg and 60 mg, respectively, for a mean duration of 24 weeks. In the overall study population, 56% were female and the average age of patients was 45 years.
Table 2 presents common adverse reactions associated with SIGNIFOR LAR in this study. These common adverse reactions were not present at baseline, or if present, worsened from baseline and occurred in at least 5% of patients treated with SIGNIFOR LAR.
Table 2 – Adverse Reactions Occurring in Greater Than or Equal to 5% of Patients Exposed to SIGNIFOR LAR in Patients With Acromegaly Previously Treated With Other Somatostatin Analogs *Diabetes mellitus includes the following PTs: diabetes mellitus and type 2 diabetes mellitus. Adverse Drug Reactions SIGNIFOR LAR 40 mg
%
N = 63SIGNIFOR LAR 60 mg
%
N = 62Active Comparators
%
N = 66Hyperglycemia Related Adverse Reactions Hyperglycemia 33 30 14 Diabetes mellitus* 21 31 9 Blood glucose increased 5 7 0 Hypoglycemia 3 7 0 Gastrointestinal Related Adverse Reactions Diarrhea 16 19 5 Abdominal pain 8 8 3 Nausea 3 7 3 Hepatobiliary Related Adverse Reactions Cholelithiasis 10 13 14 Cardiac Related Adverse Reactions Atrioventricular block first degree 6 0 0 Nervous System Related Adverse Reactions Headache 14 3 5 Dizziness 8 2 3 Skin and Subcutaneous Tissue Related Adverse Reactions Alopecia 2 7 0 Infections Related Adverse Reactions Nasopharyngitis 6 11 3 Blood Related Adverse Reactions Anemia 6 3 3 Other notable adverse reactions which occurred with a frequency of 5% or less in the SIGNIFOR LAR 40 mg, SIGNIFOR LAR 60 mg arm, respectively, were adrenal insufficiency (2% and 0%) and glucose tolerance impaired (3% and 5%).
Patients With Cushing’s Disease
The data described in Table 3 are derived from a randomized clinical study in 150 patients with Cushing’s disease [see Clinical Studies (14.3)]. These data reflect exposure of 74 and 76 patients to SIGNIFOR LAR at a starting dose of 10 mg and 30 mg once every 28 days, respectively, for a mean duration of 68 weeks. In the overall study population, 79% of patients were female and the average age was 39 years at study entry.
Table 3 presents common adverse reactions which were not present at baseline or, if present, worsened from baseline and occurred in at least 5% of patients treated with SIGNIFOR LAR.
Table 3 – Adverse Reactions Occurring in Greater Than or Equal to 5% of Patients Exposed to SIGNIFOR LAR in Patients With Cushing’s Disease *Diabetes mellitus consists of the two terms: diabetes mellitus and type 2 diabetes mellitus.
**Abdominal pain includes the term abdominal pain upper.
***Sinus bradycardia includes the term bradycardia.
****Fatigue includes the term asthenia.Adverse Reactions Overall
%
N = 150Endocrine Related Adverse Reactions Adrenal insufficiency 7 Blood cortisol decreased 5 Hyperglycemia Related Adverse Reactions Hyperglycemia 47 Diabetes mellitus* 27 Hypoglycemia 15 Blood glucose increased 9 Glycosylated hemoglobin (HbA1C) increased 5 Gastrointestinal Related Adverse Reactions Diarrhea 39 Nausea 21 Abdominal pain** 23 Constipation 7 Abdominal distension 5 Flatulence 5 Vomiting 5 Hepatobiliary Related Adverse Reactions Cholelithiasis 33 Gamma-glutamyltransferase increased 9 Alanine aminotransferase increased 7 Cardiac Related Adverse Reactions Hypertension 15 Hypotension 6 Sinus bradycardia*** 5 General Related Adverse Reactions Fatigue**** 27 Edema peripheral 14 Musculoskeletal and Connective Tissue Related Adverse Reactions Back pain 11 Arthralgia 8 Pain in extremity 7 Myalgia 5 Metabolism and Nutrition Related Adverse Reactions Decreased appetite 10 Hyperuricemia 7 Hypercholesterolemia 6 Blood Related Adverse Reactions Anemia 5 Other adverse reactions which occurred at a frequency less than 5% were cholestasis (4%), glucose tolerance impaired (3%), aspartate aminotransferase increased (3%), vomiting (3%), lipase increased (3%), injection-site reactions (2%), ECG QT prolonged (1%), cholecystitis (1%), amylase increased (1%), and prothrombin time prolonged (1%).
Hyperglycemia
The average fasting plasma glucose levels in patients with acromegaly naïve to drug therapy study [see Clinical Studies (14.1)] across visits is shown in Figure 2 below.
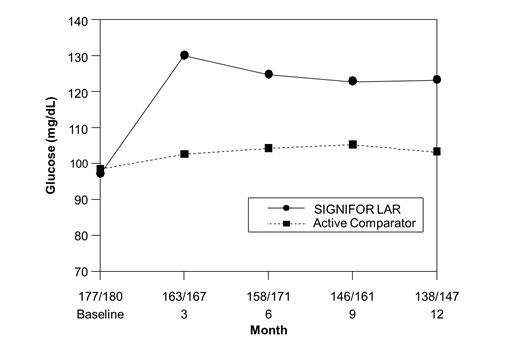
Figure 2. Mean Fasting Plasma Glucose (mg/dL) By Visit in the Study of Patients With Acromegaly Naïve to Drug Therapy*
*Numbers of patients with a glucose value at the given timepoint in the SIGNIFOR LAR/Active comparator arms are displayed as xxx/xxx on the x axis.
Pancreatic Enzyme Elevation and Pancreatitis
Asymptomatic elevations in lipase and alpha amylase were observed in 30% and 20% of patients receiving SIGNIFOR LAR in the drug naïve study in acromegaly, and in 1% and 3% of patients receiving SIGNIFOR LAR in the study of acromegaly patients previously treated. In the drug-naïve study, 2 acromegaly patients receiving SIGNIFOR LAR developed pancreatitis. In the Cushing’s disease study, increased lipase was observed in 4% of patients, and 1 patient developed pancreatitis. Pancreatitis is a potential adverse reaction associated with the use of SIGNIFOR LAR due to the association between cholelithiasis and acute pancreatitis.
6.2 Postmarketing Experience
Additional adverse reactions have been identified during postapproval use of SIGNIFOR LAR. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Cholelithiasis resulting in complications, including cholecystitis and cholangitis, which have sometimes required cholecystectomy
- Hyperglycemia and Diabetes [see Warnings and Precautions (5.1)]
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on SIGNIFOR LAR
Drugs that Prolong QT
Coadministration of drugs that prolong the QT interval with SIGNIFOR LAR may have additive effects on the prolongation of the QT interval. Monitoring effects on the QT interval at 21 days is recommended [see Warnings and Precautions (5.2)].
7.2 Effect of SIGNIFOR LAR on Other Drugs
Cyclosporine
Concomitant administration of cyclosporine with SIGNIFOR LAR may decrease the relative bioavailability of cyclosporine and, therefore, dose adjustment of cyclosporine to maintain therapeutic levels may be necessary.
Bromocriptine
Coadministration of SIGNIFOR LAR with bromocriptine may increase the blood levels of bromocriptine. Dose reduction of bromocriptine may be necessary.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited data with SIGNIFOR LAR in pregnant women are insufficient to inform a drug-associated risk for major birth defects and miscarriage. In embryo-fetal development studies in rabbits, findings indicating a developmental delay were observed with subcutaneous administration of pasireotide during organogenesis at doses less than the exposure in humans at the highest recommended dose; maternal toxicity was not observed at this dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Data
Animal Data
In embryo-fetal development studies in rats given 1 mg/kg/day, 5 mg/kg/day, and 10 mg/kg/day subcutaneously throughout organogenesis, maternal toxicity was observed at all doses, including the lowest dose tested which had exposures 12 times higher than that at the maximum therapeutic dose based on area under the curve (AUC) comparisons across species. An increased incidence of early/total resorptions and malrotated limbs was observed in rats at 10 mg/kg/day. At 10 mg/kg/day in rats, the maternal systemic exposure (AUC) was 42179 ng*hr/mL, approximately 106 times the exposure in humans at the highest recommended dose of 60 mg SIGNIFOR LAR administered as an intramuscular injection once every 4 weeks.
In embryo-fetal development studies in rabbits given 0.05 mg/kg/day, 1 mg/kg/day, and 5 mg/kg/day subcutaneously through organogenesis, maternal toxicity was observed at 1 mg/kg/day, at a maternal systemic exposure (AUC) of 1906 ng*hr/mL, approximately 5 times higher than the maximum human therapeutic exposure. An increased incidence of unossified forepaw phalanx, indicative of a developmental retardation, was observed in rabbits at 0.05 mg/kg/day, with maternal systemic exposures less than the systemic exposure in humans at the highest recommended dose.
In pre- and post-natal developmental studies in rats given subcutaneous doses of 2 mg/kg/day, 5 mg/kg/day, and 10 mg/kg/day during gestation through lactation and weaning, maternal toxicity was observed at all doses including the lowest dose (9 times higher than the maximum therapeutic dose based on surface area comparisons across species). Retardation of physiological growth, attributed to GH inhibition was observed at 2 mg/kg/day during a pre- and post-natal study in rats. After weaning, body weight gains in the rat pups (F1 generation) exposed to pasireotide were comparable to controls, showing reversibility of this developmental delay.
8.2 Lactation
Risk Summary
There is no information available on the presence of SIGNIFOR LAR in human milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. Studies show that pasireotide administered subcutaneously passes into the milk of lactating rats; however, due to species-specific differences in lactation physiology, animal data may not reliably predict drug levels in human milk (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SIGNIFOR LAR, and any potential adverse effects on the breastfed child from SIGNIFOR LAR or from the underlying maternal condition.
Data
Available data in animals have shown excretion of pasireotide in milk. After a single 1 mg/kg [14C]-pasireotide subcutaneous dose to lactating rats, the transfer of radioactivity into milk was observed. The overall milk:plasma (M/P) exposure ratio of total radioactivity was 0.28, based on AUC0-∞ values.
8.3 Females and Males of Reproductive Potential
Discuss the potential for unintended pregnancy with premenopausal women as the therapeutic benefits of a reduction in GH levels and normalization of IGF-1 in acromegalic females treated with pasireotide may lead to improved fertility.
Similarly, the therapeutic benefits of a reduction or normalization of serum cortisol levels in female patients with Cushing’s disease treated with pasireotide may also lead to improved fertility.
8.4 Pediatric Use
Safety and effectiveness of SIGNIFOR LAR have not been established in pediatric patients.
8.5 Geriatric Use
Clinical studies of SIGNIFOR LAR did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
Dose adjustment is not required in patients with mild impaired hepatic function (Child-Pugh A), but is required for patients with moderately impaired hepatic function (Child-Pugh B). The safety and efficacy of SIGNIFOR LAR have not been established in patients with severe hepatic impairment (Child-Pugh C). No dosage recommendation can be given for patients with severe hepatic impairment (Child-Pugh C) [see Dosage and Administration (2.5), Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
In the event of overdosage, it is recommended that appropriate supportive treatment be initiated, as dictated by the patient’s clinical status, until resolution of the symptoms.
Up-to-date information about the treatment of overdose can be obtained from a certified Regional Poison Center. In the event of an overdose, contact the National Capital Poison Center at 1-800-222-1222 or www.poison.org.
-
11 DESCRIPTION
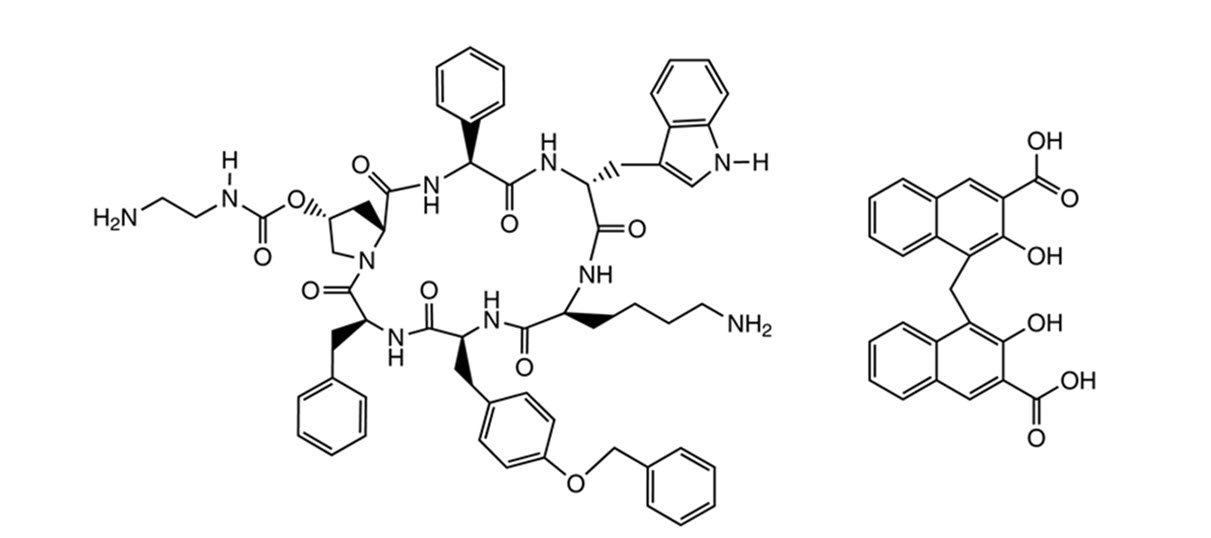
SIGNIFOR LAR (pasireotide) for injectable suspension is a long-acting release form of pasireotide pamoate, as powder to be suspended in the provided diluent immediately prior to intramuscular injection. SIGNIFOR LAR contains pasireotide, a somatostatin analog in the form of pasireotide pamoate (pamoic acid salt). Pasireotide is a cyclohexapeptide with pharmacologic properties mimicking those of the natural hormone somatostatin. Pasireotide pamoate has a chemical name of (2-Aminoethyl) carbamic acid (2R,5S,8S,11S,14R,17S,19aS)-11-(4-aminobutyl)-5-benzyl-8-(4-benzyloxybenzyl)-14-(1H-indol-3-ylmethyl)-4,7,10,13,16,19-hexaoxo-17-phenyloctadecahydro-3a,6,9,12,15,18-hexaazacyclopentacyclooctadecen-2-yl ester pamoic acid salt.
The molecular formula of pasireotide pamoate is C58H66N10O9 C23H16O6 and the molecular weight is 1435.58 g/mol.
The structural formula is:
The drug product consists of pasireotide pamoate uniformly distributed within microspheres which are made of biodegradable copolymers of poly (D,L-lactide-co-glycolide) acids (PLGA).
SIGNIFOR LAR is available in a vial containing the sterile pasireotide pamoate, PLGA microspheres powder, 10 mg, 20 mg, 30 mg, 40 mg, and 60 mg to be reconstituted with the provided 2 mL sterile diluent.
Each vial contains:
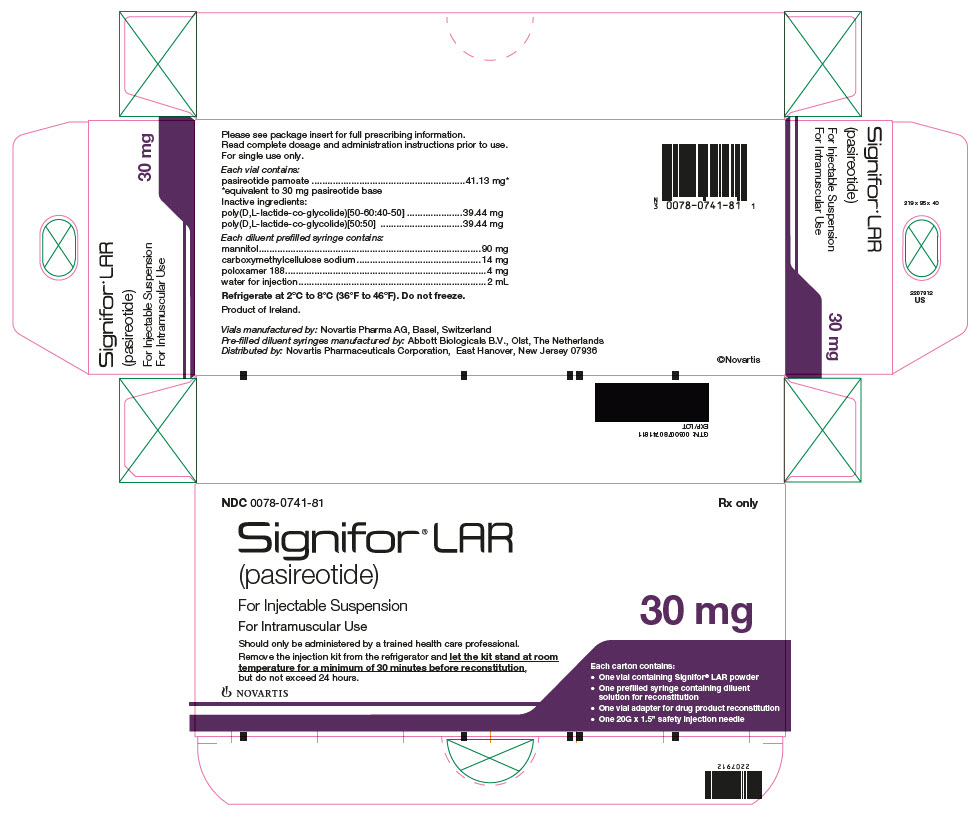
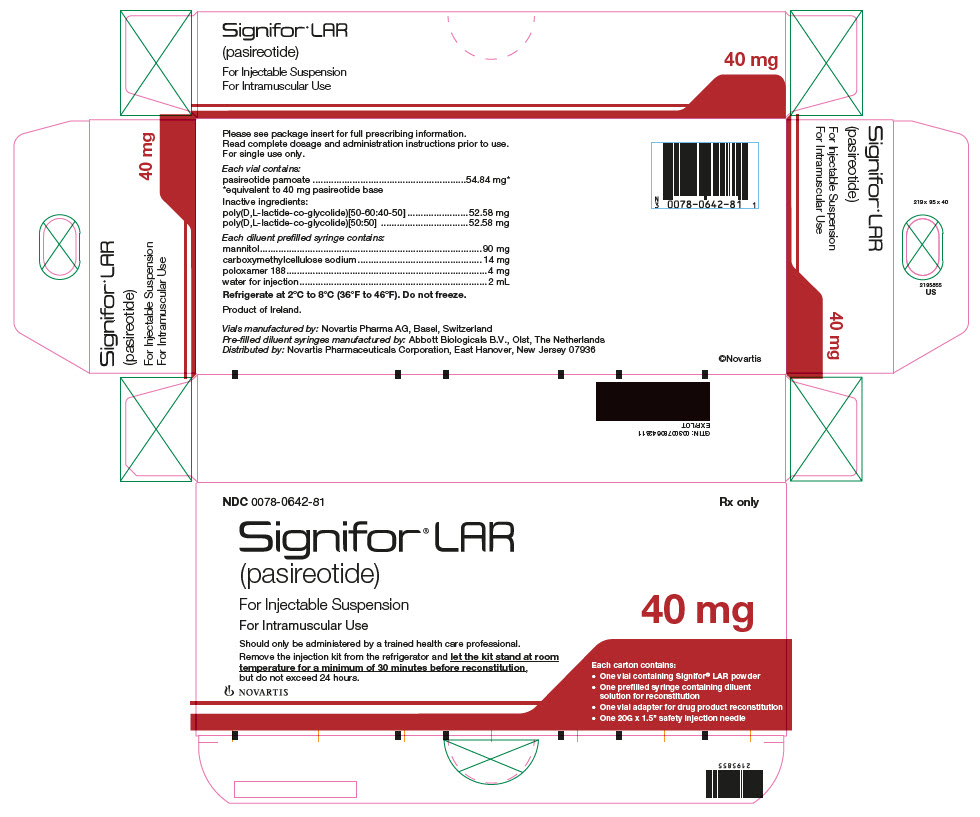
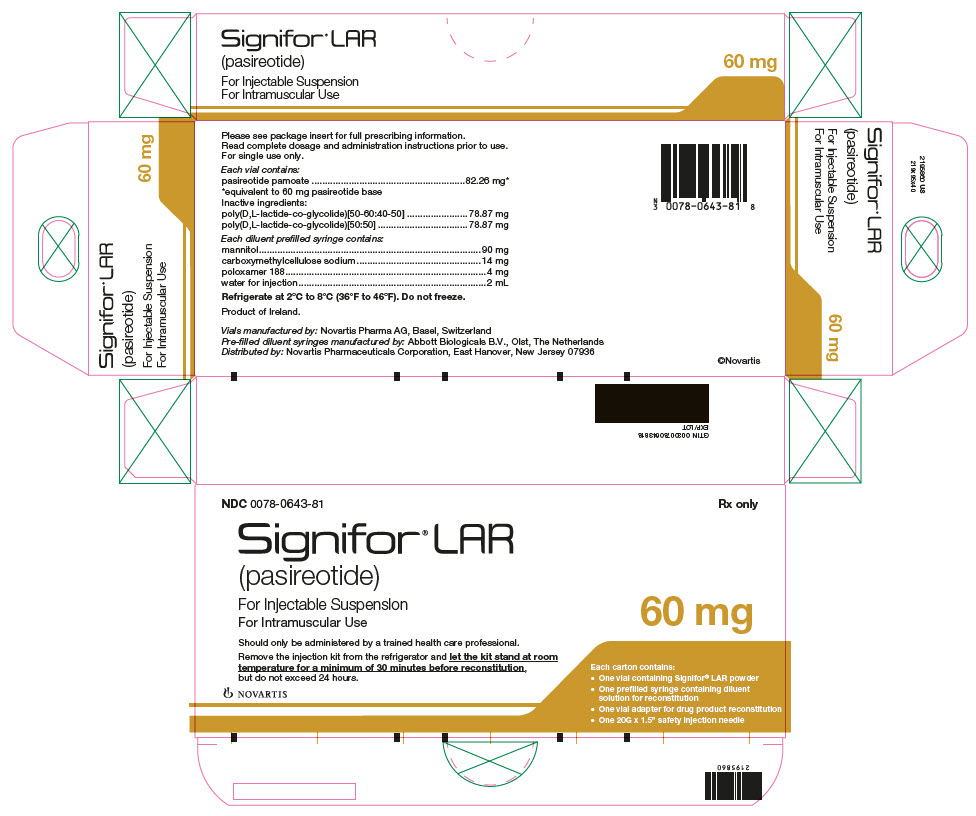
*corresponds to 10 mg, 20 mg, 30 mg, 40 mg, and 60 mg of pasireotide base, respectively. 10 mg 20 mg 30 mg 40 mg 60 mg Pasireotide pamoate 13.71 mg* 27.42 mg* 41.13 mg* 54.84 mg* 82.26 mg* Poly(D,L-lactide-co-glycolide) [50-60:40-50] 13.15 mg 26.29 mg 39.44 mg 52.58 mg 78.87 mg Poly(D,L-lactide-co-glycolide) [50:50] 13.15 mg 26.29 mg 39.44 mg 52.58 mg 78.87 mg Each diluent prefilled syringe contains:
Mannitol 90 mg Carboxymethylcellulose sodium 14 mg Poloxamer 188 4 mg Water for injections add to 2 mL -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
SIGNIFOR LAR is an injectable cyclohexapeptide, somatostatin analog. Pasireotide exerts its pharmacological activity via binding to somatostatin receptors (SSTR). There are 5 known human somatostatin receptor subtypes: SSTR 1, 2, 3, 4, and 5. These receptor subtypes are expressed in different tissues under normal physiological conditions. Somatostatin analogs bind to SSTRs with different potencies. Pasireotide binds with high affinity to 4 of the 5 SSTRs (see Table 4).
Table 4 – Binding Affinities of Somatostatin (SRIF-14) and Pasireotide to the Five Human SSTR Subtypes (SSTR 1-5) Results are the mean + SEM of IC50 values expressed as nmol/l (nM). Compound SSTR1 SSTR2 SSTR3 SSTR4 SSTR5 Somatostatin (SRIF-14) 0.93 ± 0.12 0.15 ± 0.02 0.56 ± 0.17 1.5 ± 0.4 0.29 ± 0.04 Pasireotide 9.3 ± 0.1 1.0 ± 0.1 1.5 ± 0.3 > 100 0.16 ± 0.01 12.2 Pharmacodynamics
Somatostatin receptors are expressed in many tissues including neuroendocrine tumors (e.g., growth hormone or adrenocorticotropic hormone secreting pituitary adenomas).
Acromegaly
Pasireotide binds to SSTR2 and SSTR5 subtype receptors which may be relevant for inhibition of GH secretion. In vivo studies show that SIGNIFOR LAR lowers GH and IGF-1 levels in patients with acromegaly.
Cushing’s Disease
Corticotroph tumor cells from Cushing’s disease patients frequently over-express SSTR5 whereas the other receptor subtypes are often not expressed or are expressed at lower levels. Pasireotide binds and activates the SSTRs resulting in inhibition of ACTH secretion, which leads to decreased cortisol secretion.
Cardiac Electrophysiology
Individually corrected QT (QTcI) interval was evaluated in a randomized, blinded, crossover study in healthy subjects investigating pasireotide subcutaneous doses of 0.6 mg and 1.95 mg twice daily, respectively. The maximum mean (95% upper confidence bound) placebo-subtracted QTcI change from baseline was 12.7 (14.7) ms and 16.6 (18.6) ms, respectively. Both pasireotide doses decreased heart rate, with a maximum mean (95% lower confidence bound) placebo-subtracted change from baseline of -10.9 (-11.9) beats per minute (bpm) observed at 1.5 hours for pasireotide 0.6 mg twice daily, and -15.2 (-16.5) bpm at 0.5 hours for pasireotide 1.95 mg twice daily.
The predicted pasireotide peak concentration (25.8 ng/mL) following SIGNIFOR LAR 60-mg dose in acromegaly patients is similar to the observed peak concentration (24.3 mg/mL) of the subcutaneous SIGNIFOR 0.6 mg twice daily dose and below the observed peak concentration (80.6 ng/mL) of the subcutaneous SIGNIFOR 1.95 mg twice daily dose. The predicted pasireotide peak concentration for the SIGNIFOR LAR dose of 40 mg in Cushing’s disease patients is 14 ng/mL.
12.3 Pharmacokinetics
Pasireotide for intramuscular use is formulated as microspheres for long-acting release. After a single injection, the plasma pasireotide concentration shows an initial burst release on the injection day, followed by a dip from Day 2 to Day 7, then a slow increase to the maximum concentration around Day 21, and a slow declining phase over the next weeks, concomitant with the terminal degradation phase of the polymer matrix of the dosage form.
Absorption and Distribution
No studies have been conducted to evaluate the absolute bioavailability of pasireotide in humans. Food effect is unlikely to occur since SIGNIFOR LAR is administered via a parenteral route.
In healthy volunteers, pasireotide administered as SIGNIFOR LAR is widely distributed with large apparent volume of distribution (Vz/F > 100 L). Distribution between blood and plasma is concentration-independent and shows that pasireotide is primarily located in the plasma (91%). Plasma protein binding is moderate (88%) and independent of concentration.
Pasireotide has low passive permeability and is likely to be a substrate of P-glycoprotein (P-gp), but the impact of P-gp on the ADME (absorption, distribution, metabolism, excretion) of pasireotide is expected to be low. In clinical testing in healthy volunteers, P-gp inhibition did not affect the rate or extent of pasireotide availability [see Drug Interactions (7.1)]. At therapeutic dose levels, pasireotide is not expected to be a substrate of BCRP (breast cancer resistance protein), OCT1 (organic cation transporter 1), or OATP (organic anion-transporting polypeptides) 1B1, 1B3, or 2B1.
Elimination
Metabolism and Excretion
Pasireotide was shown to be highly metabolically stable in human liver and kidney microsomes. In healthy volunteers, pasireotide in its unchanged form is the predominant form found in plasma, urine and feces. Somatropin may increase CYP450 enzymes and, therefore, suppression of GH secretion by somatostatin analogs including pasireotide may decrease the metabolic clearance of compounds metabolized by CYP450 enzymes.
Pasireotide is eliminated mainly via hepatic clearance (biliary excretion) with a small contribution of the renal route. In a human ADME study with subcutaneous SIGNIFOR with a single dose 0.6 mg, 55.9 ± 6.63% of the radioactivity dose was recovered over the first 10 days post dosing, including 48.3 ± 8.16% of the radioactivity in feces and 7.63 ± 2.03% in urine.
The apparent clearance (CL/F) of SIGNIFOR LAR in healthy volunteers is on average 4.5-8.5 L/hour.
Steady-State Pharmacokinetics
PK steady-state for SIGNIFOR LAR is achieved after 3 monthly doses. Following multiple intramuscular doses every 4 weeks (every 28 days), SIGNIFOR LAR demonstrates approximately dose-proportional PK exposures (steady-state trough; Ctrough, ss) in the dose range of 10 mg to 60 mg every 4 weeks.
Special Populations
Population PK analyses of SIGNIFOR LAR suggest that race, gender, and body weight do not have clinically relevant influence on circulating levels of pasireotide. No dose adjustment is required for demographics.
Pediatric Patients
No studies have been performed in pediatric patients [see Use in Specific Populations (8.4)].
Geriatric Patients
Age is not a significant covariate in the population PK analysis. Therefore age is not expected to significantly impact circulating levels of pasireotide.
Efficacy and safety data on patients older than 65 years are limited [see Use in Specific Populations (8.5)].
Hepatic Impairment
In a clinical study with a single subcutaneous dose of 600 µg pasireotide in subjects with impaired hepatic function (Child-Pugh A, B and C), subjects with moderate and severe hepatic impairment (Child-Pugh B and C) showed significantly higher exposures than subjects with normal hepatic function. Upon comparing with the control group, AUCinf was increased by 12%, 56%, and 42%; and Cmax was increased by 3%, 46%, and 33% respectively, in the mild, moderate, and severe hepatic impairment groups [see Use in Specific Populations (8.6), Dosage and Administration (2.5)].
Renal Impairment
Clinical studies have not been performed in patients with renal impairment. However, renal clearance has a minor contribution to the elimination of pasireotide in humans. Renal function (creatinine clearance and estimated glomerular filtration rate) is not a covariate in the population PK analysis. Therefore, renal function is not expected to significantly impact the circulating levels of pasireotide [see Use in Specific Populations (8.7)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
A lifetime carcinogenicity study was conducted in rats and transgenic mice. Rats were given daily subcutaneous doses of pasireotide at 0.01 mg/kg/day, 0.05 mg/kg/day, and 0.3 mg/kg/day for 104 weeks. There were no drug-related tumors in rats at exposures up to 5-times higher than the maximum recommended clinical exposure of the pasireotide LAR 60 mg dose. Mice were given subcutaneous doses of pasireotide at 0.5 mg/kg/day, 1 mg/kg/day, and 2.5 mg/kg/day for 26 weeks and did not identify any carcinogenic potential.
Mutagenesis
Pasireotide was not genotoxic in a battery of in vitro assays (Ames mutation test in Salmonella and Escherichia coli and mutation test in human peripheral lymphocytes). Pasireotide was not genotoxic in an in vivo rat bone marrow nucleus test.
Impairment of Fertility
Subcutaneous dosing at 0.1 mg/kg/day before mating and continuing into gestation in rats at exposures less than the human clinical exposure based on body surface area comparisons across species resulted in statistically significant increased implantation loss and decreased viable fetuses, corpora lutea, and implantation sites. Abnormal cycles or acyclicity were observed at 1 mg/kg/day (4-fold higher than the maximum therapeutic exposure of pasireotide LAR based on surface area, comparisons across species).
-
14 CLINICAL STUDIES
14.1 Drug-Naïve Patients with Acromegaly
A multicenter, randomized, double-blind study was conducted to assess the safety and efficacy of SIGNIFOR LAR in patients with active acromegaly. A total of 358 patients naïve to drugs used to treat acromegaly were randomized in a 1:1 ratio to SIGNIFOR LAR or another somatostatin analog active comparator. Randomization was stratified based on previous pituitary surgical status (e.g., at least 1 prior pituitary surgery versus no prior pituitary surgery).
In the overall study population, 52% were female and the average age of patients was 45 years. Sixty percent of patients were Caucasian, 23% Asian, 12% Other, 3% American Indian, and 2% were Black. Forty-two percent of patients had previous pituitary surgery, and 1 patient had a history of pituitary radiation therapy. Median time between diagnosis and trial participation was 6 months. Median GH was 8.8 mcg/L (range: 0.8-200 mcg/L) and 10.1 mcg/L (range: 0.6–169.6 mcg/L) for SIGNIFOR LAR and active comparator, respectively at baseline. Median standardized IGF-1, defined as IGF-1 value divided by the ULN (i.e., fold above the ULN), was 2.9 (range: 0.9–6.9) and 2.9 (range: 0.8–7.3), for SIGNIFOR LAR and active comparator, respectively, at baseline.
The starting dose of SIGNIFOR LAR was 40 mg. Dose increase was allowed in both arms, at the discretion of investigators, after 3 and 6 months of treatment if mean GH was greater than or equal to 2.5 mcg/L and/or IGF-1 was greater than the ULN for age and sex. The maximum allowed dose for SIGNIFOR LAR was 60 mg. The maximum dose of the active comparator was not used in this trial because the trial was multi-national and the maximum dose approved in the US was not approved in all participating countries.
The efficacy endpoint was the proportion of patients with a mean GH level less than 2.5 mcg/L and a normal IGF-1 levels at month 12 (age- and sex-adjusted) (see Table 5, Figure 3, and Figure 4). The proportion of patients achieving this level of control was 31.3% and 19.2% for SIGNIFOR LAR and active comparator, respectively. The changes in mean GH and IGF-1 levels by study visits in subjects with a measurement at these visits (observed cases) are shown in Figures 3 and 4.
Table 5 – Results at Month 12 in Drug-Naïve Patients Study *Primary endpoint [patients with IGF-1< lower limit of normal (LLN) were not considered as “responders”].
ULN = Upper limit of normal.
**p-value < 0.01 for treatment difference.
&The maximum dose approved for use in the United States was not used in this trial but the majority of patients were receiving the dose most commonly used in the United States to treat acromegaly.SIGNIFOR LAR
(40-60 mg)
%
N = 176Active Comparator&
%
N = 182GH < 2.5 mcg/L and normalized IGF-1* 31.3%** 19.2% GH < 2.5 mcg/L and IGF-1 ≤ ULN 35.8% 20.9% Normalized IGF-1 38.6%** 23.6% GH < 2.5 mcg/L 48.3% 51.6% 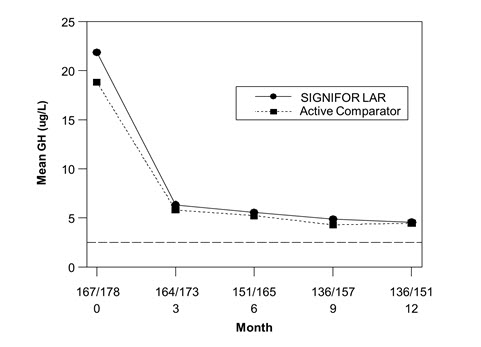
Figure 3. Mean GH (mcg/L) Levels By Visit in Drug Naïve Patient Study*
*Numbers of patients with a GH value at the given timepoint for SIGNIFOR LAR/Active comparator arm are displayed as xxx/xxx on the x-axis.
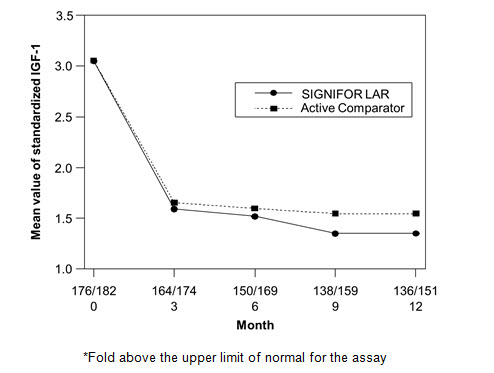
Figure 4. Mean Standardized IGF-1 Levels* By Visit in Drug Naïve Patient Study**
**Numbers of patients with an IGF-1 value at the given timepoint for SIGNIFOR LAR/Active comparator arm are displayed as xxx/xxx on the x axis.
Biochemical control was achieved by Month 3 in 30.1% of patients in the SIGNIFOR LAR arm. Ninety-eight percent of patients treated with SIGNIFOR LAR had either a reduction or no change in tumor volume from baseline assessed by MRI at Month 12. The median (range) change in tumor volume was a reduction of 39.8% (-97.6% to 16.9%).
Additionally, ring size and acromegaly symptoms score (i.e., headache, fatigue, perspiration, paresthesia, and osteoarthralgia) were followed. At Month 12, reductions in ring size and in symptom severity scores in both treatment groups compared to baseline were noted.
14.2 Patients with Acromegaly Inadequately Controlled on Other Somatostatin Analogs
A multicenter, randomized, 3-arm study was conducted in patients with acromegaly inadequately controlled on somatostatin analogs. Patients were randomized to double-blind SIGNIFOR LAR 40 mg (n = 65) or SIGNIFOR LAR 60 mg (n = 65) or to continued open-label pretrial somatostatin analog therapies at maximal or near maximal doses (n = 68). A total of 181 patients completed the 6-month trial.
Inadequate control was defined as a GH concentration of greater than 2.5 mcg/L (i.e., mean of 5 samples over 2 hours) and a sex- and age-adjusted IGF-1 level greater than 1.3 times the upper limit of normal. Patients were required to have been treated with other somatostatin analogs for at least 6 months prior to randomization. Note that the maximum dose for one of the active comparators approved for use in the United States was not used in this multinational trial; approximately 75% of the population in the comparator group was receiving this active comparator.
In the overall study population, 56% were female and the average age of patients was 45 years. Eighty-one percent of patients were Caucasian, 7% Other, 8% Black, 2% American Indian, and 2% Asian. The percentage of patients with previous pituitary surgery in the SIGNIFOR LAR 40 mg and 60 mg arms, and in the active control arm was 77%, 63%, and 60%, respectively. Three percent of patients in the SIGNIFOR LAR groups and 7% of patients in the active control arm had prior radiation therapy. Median (range) time from diagnosis to participation in this trial was 50 (10–337) months, 55 (8–357) months, and 54 (8–357) months in the SIGNIFOR LAR 40 mg, 60 mg and the pretrial therapy arms, respectively. At baseline, median (range) GH was 7.1 (1.0–200) mcg/L, 5.3 (1.4–113.8) mcg/L and 6.1 (1.0–92.4) mcg/L in the SIGNIFOR LAR 40 mg, 60 mg and the pretrial therapy arms, respectively. Baseline median standardized IGF-1 (defined as IGF-1 value divided by the ULN) values were 2.3, 2.6 and 2.9 in the SIGNIFOR LAR 40 mg, 60 mg and the pretrial therapy arms, respectively.
The efficacy endpoint was the proportion of patients with a mean GH level less than 2.5 mcg/L and normal IGF-1 levels at week 24. The primary analysis compared SIGNIFOR LAR 60 mg and 40 mg to continued pretrial therapy (i.e., no change in treatment). The proportion of patients achieving biochemical control was 15.4% and 20.0% for SIGNIFOR LAR 40 mg and 60 mg, respectively, at 6 months.
Biochemical control was achieved by Month 3 in 15.4% and 18.5% of patients in the SIGNIFOR LAR 40 mg and 60 mg arms, respectively.
Table 6 – Results at 6 Months in Inadequately Controlled Patient Study *Primary endpoint (patients with IGF-1 < lower limit of normal (LLN) were not considered as “responders”).
&For one of the active comparators, the maximum dose approved for use in the United States was not used in this trial but the majority of patients were receiving the dose most commonly used in the United States to treat acromegaly.SIGNIFOR LAR 40 mg
%
N = 65SIGNIFOR LAR 60 mg
%
N = 65Continued
Pre-Trial Therapy
Control Arm&
%
N = 68GH < 2.5 mcg/L and normalized IGF-1* 15.4% 20.0% 0% Normalization of IGF-1 24.6% 26.2% 0% GH < 2.5 mcg/L 35.4% 43.1% 13.2% Eighty-one percent and 70% of patients treated with SIGNIFOR LAR 40 mg and 60 mg, respectively, had either a reduction or no change in tumor volume from baseline assessed by MRI at Month 6. The median (range) change in tumor volume was a reduction of -10.4% (-74.5% to 19.4%) and -6.3% (-66.7% to 14.5%) from baseline for SIGNIFOR LAR 40 mg and 60 mg, respectively.
14.3 Patients with Cushing’s Disease
A Phase 3, randomized, double-blind, multicenter study was conducted to evaluate the safety and efficacy of two dose regimens of SIGNIFOR LAR over a 12-month treatment period in patients with persistent or recurrent Cushing’s disease, or de novo patients who were not considered candidates for pituitary surgery.
The study enrolled 150 patients with a screening mean urinary free cortisol level (mUFC) ≥ 1.5 and ≤ 5 x ULN, who were randomized in a 1:1 ratio to receive a SIGNIFOR LAR starting dose of either 10 mg intramuscularly once every 28 days or 30 mg intramuscularly once every 28 days. Randomization was stratified by values of mUFC at screening (1.5 to < 2 x ULN versus 2 to 5 x ULN, respectively).
After four months of treatment, patients who had a mUFC ≤ 1.5 x ULN continued on the blinded dose to which they were randomized. Patients with a mUFC > 1.5 x ULN at Month 4 had their doses increased in a blinded manner from 10 mg to 30 mg, or from 30 mg to 40 mg, provided there were no tolerability concerns. Additional dose increases were allowed at Month 7 and Month 9 (by one dose level if the mUFC was > 1 x ULN).
Dose reduction by one dose level for tolerability was allowed in a blinded fashion for the first seven months, with a minimum dose level of 5 mg. After the first seven months, blinded down titration of more than one dose level was allowed at any month.
After twelve months of treatment (core phase), patients had the option to enter an extension to continue to receive SIGNIFOR LAR if they benefited from treatment.
The primary efficacy endpoint was the proportion of patients in each arm who were mUFC responders (mUFC ≤ ULN) after seven months of treatment, with or without up-titration at Month 4. The key secondary endpoint was the proportion of patients in each arm who were mUFC responders after seven months of treatment and who did not up-titrate the dose prior to Month 7. The pre-specified boundary of the lower limit of the 95% confidence interval (CI) for efficacy for both the primary and key secondary endpoints was 15%. Patients with missing mUFC assessment at Month 7 were classified as non-responders. Other secondary endpoints included changes from baseline in 24-hour UFC, plasma ACTH, and serum cortisol levels. All analyses were conducted based on the randomized dose groups.
Baseline demographics and disease history were well balanced between the two randomized dose groups and consistent with the epidemiology of the disease. The mean age of patients was approximately 38.5 years with a predominance of female patients (78.7%). The majority of the patients had persistent or recurrent Cushing’s disease (82.0%). The median value of the baseline 24-hour mUFC for all patients was 396.9 nmol/24 hours (ULN: 166.5 nmol/24 hours). About three-quarters of all randomized patients completed seven months of treatment, and about two-thirds of all randomized patients completed twelve months of treatment.
The study met the primary efficacy objective for both dose groups. Patients were considered responders if they remained on treatment until at least Month 7 and achieved a Month 7 mUFC ≤ 1 x ULN, regardless of up-titration at Month 4. The proportion of patients with mUFC response at Month 7 was 39.2% (95% CI: 28.0, 51.2) in the 10 mg arm and 40.8% (95% CI: 29.7, 52.7) in the 30 mg arm. The responder rate at Month 12 was 35.1% (26/74) and 25.0% (19/76) in the 10 mg and 30 mg starting dose groups, respectively.
Table 7 – Response Rates at Month 7 per Randomized Dose Group and According to Screening mUFC - There were 17 (23.0%) patients in 10 mg arm and 9 (11.8%) in the 30 mg arm with missing value mUFC assessments at Month 7 who were classified as non-responders. Pasireotide LAR
10 mg Every 28 DaysPasireotide LAR
30 mg Every 28 DaysScreening mUFC category n/N (%)
95% CIn/N (%)
95% CIAll patients 29/74 (39.2)
(28.0, 51.2)31/76 (40.8)
(29.7, 52.7)≥ 1.5 x ULN to < 2 x ULN 11/25 (44.0)
(24.4, 65.1)13/25 (52.0)
(31.3, 72.2)≥ 2 x ULN to ≤ 5 x ULN 18/49 (36.7)
(23.4, 51.7)18/51 (35.3)
(22.4, 49.9)The study met the key secondary efficacy objective for both dose groups. At Month 4, 31/74 (41.9%) and 28/76 (36.8%) patients were up-titrated in the pasireotide LAR 10 mg and 30 mg arms, respectively. When all patients who up-titrated prior to Month 7 were counted as non-responders, Month 7 mUFC response was observed in 25.7% (95% CI: 16.2 to 37.2) and 31.6% (95% CI: 21.4 to 43.3) of patients randomized to pasireotide LAR at a starting dose of 10 mg once every 28 days and 30 mg once every 28 days, respectively.
A secondary efficacy analysis was conducted for the combined proportion of patients who attained mUFC ≤ ULN (controlled) or had at least 50% reduction in mUFC (partially controlled) in the core phase of the study. The combined rate of controlled or partially controlled responders at Month 7 constituted 44.6% and 53.9% of patients randomized to the 10 mg and 30 mg dose groups, respectively (Table 8).
Table 8 – Response Rates at Month 7 per Randomized Dose Group - Supportive Efficacy Analysis Response Category Pasireotide LAR
10 mg Every 28 Days
(N = 74)
n (%)Pasireotide LAR
30 mg Every 28 Days
(N = 76)
n (%)Controlled (mUFC ≤ ULN) 29 (39.2%) 31 (40.8%) Partially controlled (≥ 50% reduction in mUFC) 4 (5.4%) 10 (13.2%) Combined 33 (44.6%) 41 (53.9%) Decreases in median mUFC levels at Month 7 compared to baseline, as measured by overall percentage of reduction, are shown in Table 9. Reductions in serum cortisol and plasma ACTH levels were also observed at Months 7 and 12 for each dose group.
Table 9 – Median Percentage Change from Baseline in Mean Urinary Free Cortisol (mUFC) at Month 7 by Randomized Dose Group Note: Median % change from baseline in mUFC at Month 7 are calculated by imputing missing values with the worst observed % change in mUFC at Month 7 within each treatment group. Pasireotide LAR 10 mg Every 28 Days
% changePasireotide LAR 30 mg Every 28 Days
% changemUFC levels (nmol/24hr)
BaselineN
Mean (SD)
Median74
462.6 (256.41)
409.876
477.1 (331.75)
371.6Median change in mUFC (% from baseline) Month 7 -41.3% -41.4% -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
SIGNIFOR LAR for injectable suspension is supplied in a single-use kit containing the following:
- One 6-mL brownish glass vial with a grey rubber stopper of SIGNIFOR LAR containing slightly yellow to yellow powder with a flip-off cap
- One 3-mL glass barrel/grey rubber stopper prefilled syringe containing 2 mL of clear, colorless to slightly yellow/brown diluent solution for reconstitution
- One sterile 20G x 1.5” stainless steel, polypropylene safety injection needle
- One vial adapter made of polycarbonate for drug product reconstitution
SIGNIFOR LAR kits are available in the following strengths:
SIGNIFOR LAR Kit Final Concentration When Reconstituted
(total product strength per total volume)Final Concentration When Reconstituted
(per mL)Flip-off Cap Color NDC 10 mg 10 mg/2 mL 5 mg/mL Brown 0078-0748-81 20 mg 20 mg/2 mL 10 mg/mL Gray 0078-0641-81 30 mg 30 mg/2 mL 15 mg/mL Lilac 0078-0741-81 40 mg 40 mg/2 mL 20 mg/mL Red 0078-0642-81 60 mg 60 mg/2 mL 30 mg/mL Orange 0078-0643-81 16.2 Storage and Handling
Store at 2°C to 8°C (36°F-46°F). Do not freeze.
SIGNIFOR LAR should be stored at refrigerated temperatures between 2°C to 8°C (36°F-46°F) until the time of use. SIGNIFOR LAR drug product kit should remain at room temperature for a minimum of 30 minutes before reconstitution, but should not exceed 24 hours at room temperature. However, after preparation of the drug suspension, it must be administered immediately.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Instruct patients on the importance of adhering to their return visit schedule.
- Advise patients that SIGNIFOR LAR should only be administered by a trained healthcare professional.
Hyperglycemia and Diabetes
- Advise patients to assess fasting plasma glucose and HbA1c prior to starting treatment with SIGNIFOR LAR and advise patients to consistently monitor blood glucose levels, particularly after the start of treatment or after dose changes, so that appropriate action can be taken [see Warnings and Precautions (5.1)].
Bradycardia and QT Prolongation
- Advise patients that an ECG will be taken before treatment and periodically thereafter. Advise patients with cardiac disease and with risk factors for QT prolongation and bradycardia that adjustments in cardiac medications may be made and electrolyte disturbances may require correction [see Warnings and Precautions (5.2)].
Liver Test Elevations
- Advise patients that liver function will be assessed prior to starting treatment with SIGNIFOR LAR and will be closely monitored for the first three months of treatment and thereafter as clinically indicated [see Warnings and Precautions (5.3)].
Cholelithiasis and Complications of Cholelithiasis
- Inform patients that cholelithiasis and complications of cholelithiasis have been reported with the use of SIGNIFOR LAR [see Warnings and Precautions (5.4)].
- Advise patients to contact their healthcare provider if they experience signs or symptoms of gallstones (cholelithiasis) or complications of cholelithiasis (e.g., cholecystitis or cholangitis) [see Warnings and Precautions (5.4)].
- Advise patients that the gallbladder will be monitored by ultrasound periodically [see Warnings and Precautions (5.4)].
Pituitary Hormone Deficiency(ies)
- Advise patients that monitoring of anterior pituitary function will be performed periodically [see Warnings and Precautions (5.5)].
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, NJ 07936T2019-53
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 4/2019 Patient Information
SIGNIFOR® LAR (sig-na-for L.A.R.)
(pasireotide)
for injectable suspension, for intramuscular useRead this Patient Information before you start receiving SIGNIFOR LAR, and each time you receive it. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is SIGNIFOR LAR?
SIGNIFOR LAR is a prescription medicine used to treat people with:
- acromegaly for whom surgery has not worked well enough or who cannot have surgery.
- Cushing’s disease for whom surgery has not worked well enough or who cannot have surgery.
It is not known if SIGNIFOR LAR is safe and effective for use in children.
What should I tell my healthcare provider before receiving SIGNIFOR LAR?
Before you receive SIGNIFOR LAR, tell your healthcare provider about all of your medical conditions, including if you:
- have high blood sugar (hyperglycemia).
- have diabetes.
- have or have had heart problems, including an abnormal heart rate or rhythm or problems with the electrical system of your heart (QT prolongation).
- have a low level of potassium or magnesium in your blood.
- have liver problems.
- have gallstones (cholelithiasis).
- are pregnant or plan to become pregnant. It is not known if SIGNIFOR LAR will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if SIGINFOR LAR passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take SIGNIFOR LAR.
Treatment with SIGNIFOR LAR may result in improved fertility and the possibility of unplanned pregnancy in females who have acromegaly or Cushing’s disease and have not gone through menopause. Talk to your healthcare provider about birth control methods that may be right for you during treatment with SIGNIFOR LAR.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
SIGNIFOR LAR and other medicines may affect each other, causing side effects. SIGNIFOR LAR may affect the way other medicines work, and other medicines may affect how SIGNIFOR LAR works. Your healthcare provider may need to change your dose of SIGNIFOR LAR or your other medicines. Especially tell your healthcare provider if you take:- medicines to control your heart beat (antiarrhythmics)
- medicines to control your blood pressure (such as beta-blockers or calcium channel blockers)
- medicines to control the potassium and magnesium (electrolytes) levels in your body
- medicines that may affect the way the electrical system of your heart works (QT prolongation)
- cyclosporine
- bromocriptine
Ask your healthcare provider for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.How will I receive SIGNIFOR LAR?
- SIGNIFOR LAR must be given by a trained healthcare provider as an injection into the muscle of your buttocks (intramuscularly).
- Your healthcare provider will tell you how much SIGNIFOR LAR you will receive and when you will receive it.
- Your healthcare provider may change your dose of SIGNIFOR LAR or the length of time between your injections. Your healthcare provider will tell you how long you need to receive SIGNIFOR LAR.
- Before you receive SIGNIFOR LAR for the first time, your healthcare provider should do a blood test to check your fasting blood sugar level, hemoglobin A1c level, electrolyte levels, and your liver function.
- You will need to check your blood sugar levels during treatment with SIGNIFOR LAR, especially after you start treatment with SIGNIFOR LAR and after your dose is increased. Your healthcare provider will tell you how often you should check your blood sugar levels.
- Before you receive SIGNIFOR LAR for the first time and during your treatment, your healthcare provider should do a test to check your heart (electrocardiogram).
- If you miss a dose of SIGNIFOR LAR, you may receive your missed dose up to 14 days before your next dose.
- It is important that you keep your scheduled appointments with your healthcare provider during treatment with SIGNIFOR LAR.
What are the possible side effects of SIGNIFOR LAR?
SIGNIFOR LAR may cause serious side effects, including:
- high blood sugar (hyperglycemia) and diabetes. High blood sugar and diabetes are serious but common side effects of SIGNIFOR LAR. Your healthcare provider should check your blood sugar level before you start receiving SIGNIFOR LAR and while you receive it. Tell your healthcare provider if you have any of these symptoms:
o feel very thirsty
o urinate more than usualo increased appetite with weight loss
o tirednessIf you get hyperglycemia while receiving SIGNIFOR LAR, your healthcare provider may give you another medicine to lower your blood sugar. Your healthcare provider may also change your dose of SIGNIFOR LAR or advise you to stop receiving it.
- slow heart rate (bradycardia). SIGNIFOR LAR can cause your heart to beat slower. People who have, or have had heart problems, or take certain medicines used to treat slow heart rate or that may cause a slow heart rate, are at higher risk for bradycardia. Tell your healthcare provider if you get any of these symptoms:
o weakness
o dizzinesso fainting or near fainting spells
- changes in the electrical system of your heart (QT interval prolongation). Tell your healthcare provider if you get any of these symptoms:
o weakness
o dizzinesso fainting or near fainting spells
- higher than normal liver function tests. Your healthcare provider should do blood tests to check your liver while you receive SIGNIFOR LAR.
- gallstones (cholelithiasis) and complications that can happen if you have gallstones. Gallstones are a serious but common side effect of SIGNIFOR LAR. Possible complications of gallstones include inflammation and infection of the gall bladder. Your healthcare provider should do a test (ultrasound) to check your gall bladder before and during your treatment with SIGNIFOR LAR. Tell your healthcare provider if you get any of these symptoms:
o sudden pain in your upper right stomach area (abdomen)
o sudden pain in your right shoulder or between your shoulder bladeso yellowing of your skin and whites of your eyes
o fever with chills
o nausea
- low levels of pituitary hormones (pituitary insufficiency). SIGNIFOR LAR may reduce the pituitary hormones in your body. Your healthcare provider should do a blood test to check your pituitary hormone levels before you start receiving SIGNIFOR LAR and while you receive it. Tell your healthcare provider if you get any of these symptoms:
o nausea and vomiting
o tiredness
o dizziness
o diarrheao low blood sugar levels
o loss of appetite
o weight lossThe most common side effects of SIGNIFOR LAR include:
diarrhea
headache
stomach-area pain
hair loss
stuffy nose and sore throat
low blood sugar
limb swelling
loss of appetitenausea
increase in the level of an enzyme in your blood called creatine phosphokinase (CPK)
tiredness
stomach bloating
high blood pressure
back painTell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of SIGNIFOR LAR. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1–800–FDA–1088.
How should I store SIGNIFOR LAR?
- Store SIGNIFOR LAR in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze SIGNIFOR LAR.
- Take SIGNIFOR LAR out of the refrigerator at least 30 minutes before you will receive it to allow it to come to room temperature.
- Do not use SIGNIFOR LAR if it has been out of the refrigerator and at room temperature for more than 24 hours.
- Your healthcare provider should give you SIGNIFOR LAR right away after it is mixed.
Keep SIGNIFOR LAR and all medicines out of the reach of children.
General information about the safe and effective use of SIGNIFOR LAR.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use SIGNIFOR LAR for a condition for which it was not prescribed. Do not give SIGNIFOR LAR to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about SIGNIFOR LAR. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about SIGNIFOR LAR that is written for health professionals.
For more information go to www.SIGNIFORLAR.com or call 1-888-NOW-NOVA.
What are the ingredients in SIGNIFOR LAR?
Active ingredient: pasireotide pamoate
Inactive ingredients:
Vial: Poly(D,L-lactide-co-glycolide)
Prefilled syringe: Mannitol, carboxymethylcellulose sodium, poloxamer 188, and water for injections
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, NJ 07936
T2019-54
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Package Label – 10 mg
Rx Only NDC: 0078-0748-81
Signifor LAR (pasireotide)
For Injectable Suspension
For Intramuscular Use
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Package Label – 20 mg
Rx Only NDC: 0078-0641-81
Signifor LAR (pasireotide)
For Injectable Suspension
For Intramuscular Use
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Package Label – 30 mg
Rx Only NDC: 0078-0741-81
Signifor LAR (pasireotide)
For Injectable Suspension
For Intramuscular Use
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Package Label – 40 mg
Rx Only NDC: 0078-0642-81
Signifor LAR (pasireotide)
For Injectable Suspension
For Intramuscular Use
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Package Label – 60 mg
Rx Only NDC: 0078-0643-81
Signifor LAR (pasireotide)
For Injectable Suspension
For Intramuscular Use
-
INGREDIENTS AND APPEARANCE
SIGNIFOR LAR
pasireotide kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-0748 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0748-81 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 06/29/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 6 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 SIGNIFOR LAR
pasireotide injection, powder, for suspensionProduct Information Item Code (Source) NDC: 0078-0755 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PASIREOTIDE (UNII: 98H1T17066) (PASIREOTIDE - UNII:98H1T17066) PASIREOTIDE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLY(DL-LACTIC-CO-GLYCOLIC ACID), (50:50; 63000 MW) (UNII: QD923V64H3) POLY(DL-LACTIC-CO-GLYCOLIC ACID), (50:50; 12000 MW) (UNII: WE369X5600) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0755-61 6 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203255 07/12/2018 Part 2 of 2 DILUENT
diluent injection, solutionProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 45 mg in 1 mL CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) 7 mg in 1 mL POLOXAMER 188 (UNII: LQA7B6G8JG) 2 mg in 1 mL WATER (UNII: 059QF0KO0R) 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203255 07/12/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203255 12/15/2014 SIGNIFOR LAR
pasireotide kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-0641 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0641-81 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 12/15/2014 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 6 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 SIGNIFOR LAR
pasireotide injection, powder, for suspensionProduct Information Item Code (Source) NDC: 0078-0762 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PASIREOTIDE (UNII: 98H1T17066) (PASIREOTIDE - UNII:98H1T17066) PASIREOTIDE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLY(DL-LACTIC-CO-GLYCOLIC ACID), (50:50; 63000 MW) (UNII: QD923V64H3) POLY(DL-LACTIC-CO-GLYCOLIC ACID), (50:50; 12000 MW) (UNII: WE369X5600) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0762-61 6 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203255 12/15/2014 Part 2 of 2 DILUENT
diluent injection, solutionProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 45 mg in 1 mL CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) 7 mg in 1 mL POLOXAMER 188 (UNII: LQA7B6G8JG) 2 mg in 1 mL WATER (UNII: 059QF0KO0R) 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203255 12/15/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203255 12/15/2014 SIGNIFOR LAR
pasireotide kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-0741 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0741-81 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 06/29/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 6 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 SIGNIFOR LAR
pasireotide injection, powder, for suspensionProduct Information Item Code (Source) NDC: 0078-0769 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PASIREOTIDE (UNII: 98H1T17066) (PASIREOTIDE - UNII:98H1T17066) PASIREOTIDE 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLY(DL-LACTIC-CO-GLYCOLIC ACID), (50:50; 63000 MW) (UNII: QD923V64H3) POLY(DL-LACTIC-CO-GLYCOLIC ACID), (50:50; 12000 MW) (UNII: WE369X5600) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0769-61 6 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203255 07/12/2018 Part 2 of 2 DILUENT
diluent injection, solutionProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 45 mg in 1 mL CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) 7 mg in 1 mL POLOXAMER 188 (UNII: LQA7B6G8JG) 2 mg in 1 mL WATER (UNII: 059QF0KO0R) 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203255 07/12/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203255 12/15/2014 SIGNIFOR LAR
pasireotide kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-0642 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0642-81 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 12/15/2014 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 6 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 SIGNIFOR LAR
pasireotide injection, powder, for suspensionProduct Information Item Code (Source) NDC: 0078-0776 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PASIREOTIDE (UNII: 98H1T17066) (PASIREOTIDE - UNII:98H1T17066) PASIREOTIDE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLY(DL-LACTIC-CO-GLYCOLIC ACID), (50:50; 63000 MW) (UNII: QD923V64H3) POLY(DL-LACTIC-CO-GLYCOLIC ACID), (50:50; 12000 MW) (UNII: WE369X5600) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0776-61 6 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203255 12/15/2014 Part 2 of 2 DILUENT
diluent injection, solutionProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 45 mg in 1 mL CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) 7 mg in 1 mL POLOXAMER 188 (UNII: LQA7B6G8JG) 2 mg in 1 mL WATER (UNII: 059QF0KO0R) 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203255 12/15/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203255 12/15/2014 SIGNIFOR LAR
pasireotide kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0078-0643 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0643-81 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 12/15/2014 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, SINGLE-USE 6 mL Part 2 1 SYRINGE 2 mL Part 1 of 2 SIGNIFOR LAR
pasireotide injection, powder, for suspensionProduct Information Item Code (Source) NDC: 0078-0783 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PASIREOTIDE (UNII: 98H1T17066) (PASIREOTIDE - UNII:98H1T17066) PASIREOTIDE 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLY(DL-LACTIC-CO-GLYCOLIC ACID), (50:50; 63000 MW) (UNII: QD923V64H3) POLY(DL-LACTIC-CO-GLYCOLIC ACID), (50:50; 12000 MW) (UNII: WE369X5600) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0078-0783-61 6 mL in 1 VIAL, SINGLE-USE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203255 12/15/2014 Part 2 of 2 DILUENT
diluent injection, solutionProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 45 mg in 1 mL CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) 7 mg in 1 mL POLOXAMER 188 (UNII: LQA7B6G8JG) 2 mg in 1 mL WATER (UNII: 059QF0KO0R) 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203255 12/15/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203255 12/15/2014 Labeler - Novartis Pharmaceuticals Corporation (002147023)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.