CIPROFLOXACIN AND DEXAMETHASONE suspension/ drops
Ciprofloxacin and Dexamethasone by
Drug Labeling and Warnings
Ciprofloxacin and Dexamethasone by is a Prescription medication manufactured, distributed, or labeled by Amneal Pharmaceuticals of New York LLC, Amneal Pharmaceuticals Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CIPROFLOXACIN AND DEXAMETHASONE OTIC SUSPENSION safely and effectively. See full prescribing information for CIPROFLOXACIN AND DEXAMETHASONE OTIC SUSPENSION.
CIPROFLOXACIN AND DEXAMETHASONE otic suspension
Initial U.S. Approval: 2003INDICATIONS AND USAGE
Ciprofloxacin and dexamethasone otic suspension is a combination of ciprofloxacin, a fluoroquinolone antibacterial and dexamethasone, a corticosteroid, indicated for the treatment of infections caused by susceptible isolates of the designated microorganisms in the specific condition listed below:
- Acute Otitis Externa (AOE) in pediatric (age 6 months and older), adult, and elderly patients due to Staphylococcus aureus and Pseudomonas aeruginosa. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Otic Suspension: Each mL of ciprofloxacin and dexamethasone otic suspension, USP contains ciprofloxacin hydrochloride USP, 0.3% (equivalent to 3 mg ciprofloxacin base) and dexamethasone USP, 0.1% (equivalent to 1 mg dexamethasone, USP). (3)
CONTRAINDICATIONS
- Ciprofloxacin and dexamethasone otic suspension is contraindicated in patients with a history of hypersensitivity to ciprofloxacin, to other quinolones, or to any of the components in this medication. (4)
- Use of this product is contraindicated in viral infections of the external canal, including herpes simplex infections and fungal otic infections. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions were ear discomfort (3%), ear pain (2.3%), and ear pruritus (1.5%). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Potential for Microbial Overgrowth with Prolonged Use
5.3 Continued or Recurrent Otorrhea
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Ciprofloxacin and dexamethasone otic suspension is indicated for the treatment of infections caused by susceptible isolates of the designated microorganisms in the specific condition listed below:
- Acute Otitis Externa (AOE) in pediatric (age 6 months and older), adult and elderly patients due to Staphylococcus aureus and Pseudomonas aeruginosa.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- Ciprofloxacin and dexamethasone otic suspension is for otic use (ears) only, and not for ophthalmic use, or for injection.
- Shake well immediately before use.
2.2 Dosage
For the Treatment of Acute Otitis Externa (age 6 months and older)
The recommended dosage regimen is as follows:
- Four drops [equivalent to 0.14 mL of ciprofloxacin and dexamethasone otic suspension, (consisting of 0.42 mg ciprofloxacin and 0.14 mg dexamethasone)] instilled into the affected ear twice daily for seven days.
- The suspension should be warmed by holding the bottle in the hand for one or two minutes to avoid dizziness, which may result from the instillation of a cold suspension.
- The patient should lie with the affected ear upward, and then the drops should be instilled.
- This position should be maintained for 60 seconds to facilitate penetration of the drops into the ear canal. Repeat, if necessary, for the opposite ear.
- Discard unused portion after therapy is completed.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- Ciprofloxacin and dexamethasone otic suspension is contraindicated in patients with a history of hypersensitivity to ciprofloxacin, to other quinolones, or to any of the components in this medication.
- Use of this product is contraindicated in viral infections of the external canal, including herpes simplex infections and fungal otic infections.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Ciprofloxacin and dexamethasone otic suspension should be discontinued at the first appearance of a skin rash or any other sign of hypersensitivity. Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving systemic quinolones. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, angioedema (including laryngeal, pharyngeal, or facial edema), airway obstruction, dyspnea, urticaria, and itching.
5.2 Potential for Microbial Overgrowth with Prolonged Use
Prolonged use of ciprofloxacin and dexamethasone otic suspension may result in overgrowth of non-susceptible bacteria and fungi. If the infection is not improved after one week of treatment, cultures should be obtained to guide further treatment. If such infections occur, discontinue use and institute alternative therapy.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)].
- Potential for Microbial Overgrowth with Prolonged Use [see Warnings and Precautions (5.2)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In Phases II and III clinical trials, a total of 537 patients were treated with ciprofloxacin and dexamethasone otic suspension with AOE. The reported adverse reactions are listed below:
Acute Otitis Externa
The following adverse reactions occurred in 0.4% or more of the patients with intact tympanic membranes.
Adverse Reactions
Incidence (N = 537)
Ear pruritus
1.5%
Ear debris
0.6%
Superimposed ear infection
0.6%
Ear congestion
0.4%
Ear pain
0.4%
Erythema
0.4%
The following adverse reactions were each reported in a single patient: ear discomfort; decreased hearing; and ear disorder (tingling).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of ciprofloxacin and dexamethasone otic suspension. Because these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reactions include auricular swelling, headache, hypersensitivity, otorrhea, skin exfoliation, rash erythematous, and vomiting.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on ciprofloxacin and dexamethasone otic suspension use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Because of the minimal systemic absorption of ciprofloxacin and dexamethasone following topical otic administration of ciprofloxacin and dexamethasone otic suspension, this product is expected to be of minimal risk for maternal and fetal toxicity when administered to pregnant women [see Clinical Pharmacology (12.3)].
Animal reproduction studies have not been conducted with ciprofloxacin and dexamethasone otic suspension. Oral administration of ciprofloxacin during organogenesis at doses up to 100 mg/kg to pregnant mice and rats, and up to 30 mg/kg to pregnant rabbits did not cause fetal malformations (see Data). These doses were at least 200 times the recommended otic human dose (ROHD in mice, rats, and rabbits, respectively, based on body surface area (BSA). With dexamethasone, malformations have been observed in animal studies after ocular and systemic administration.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and of miscarriage is 15% to 20% respectively.
Data
Animal Data
Ciprofloxacin
Developmental toxicology studies have been performed with ciprofloxacin in rats, mice, and rabbits. The doses used in these studies are, at a minimum, approximately 200 times greater than the recommended otic human dose based on body surface area. In rats and mice, oral doses up to 100 mg/kg administered during organogenesis (Gestation Days (GD), 6 to 17) were not associated with adverse developmental outcomes, including embryo-fetal toxicity or malformations. A 30 mg/kg oral dose was associated with suppression of maternal and fetal body weight gain, but fetal malformations were not observed. Intravenous administration of doses up to 20 mg/kg to pregnant rabbits was not maternally toxic and neither embryofetal toxicity nor fetal malformations were observed. To mitigate maternal toxicity in these studies, groups of rabbits received ciprofloxacin for a different 5 day dosing period covering organogenesis (GD 6 to 18).
Dexamethasone
Dexamethasone has been shown to be teratogenic in mice and rabbits following topical ophthalmic application. In a rat oral developmental toxicity study, no adverse effects were observed at 0.01 mg/kg/day (0.1 times the ROHD based on BSA), although embryotoxicity was observed at higher doses.
8.2 Lactation
Risk Summary
It is not known whether ciprofloxacin and dexamethasone are present in human milk following topical otic administration.
Published literature reports the presence of ciprofloxacin in human milk after oral administration to lactating women. However, because of the minimal systemic absorption of ciprofloxacin following topical otic administration of ciprofloxacin and dexamethasone otic suspension, breastfeeding is not expected to result in the exposure of the infant to ciprofloxacin [see Clinical Pharmacology (12.3)].
Systemically administered corticosteroids appear in human milk. Dexamethasone in breast milk could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. However, it is not known whether topical otic administration of ciprofloxacin and dexamethasone otic suspension could result in systemic absorption that is sufficient to produce detectable quantities of dexamethasone in human milk.
There are no data on the effects of ciprofloxacin or dexamethasone on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ciprofloxacin and dexamethasone otic suspension and any potential adverse effects on the breast-fed child from ciprofloxacin and dexamethasone otic suspension.
8.4 Pediatric Use
The safety and efficacy of ciprofloxacin and dexamethasone otic suspension have been established in pediatric patients 6 months and older (537 patients) in adequate and well-controlled clinical trials.
No clinically relevant changes in hearing function were observed in 69 pediatric patients (age 4 to 12 years) treated with ciprofloxacin and dexamethasone otic suspension and tested for audiometric parameters.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Ciprofloxacin and Dexamethasone Otic Suspension, USP contains the quinolone antimicrobial, ciprofloxacin hydrochloride, USP, combined with the corticosteroid, dexamethasone USP, in a sterile, preserved suspension for otic use. Each mL of ciprofloxacin and dexamethasone otic suspension, USP contains ciprofloxacin hydrochloride, USP (equivalent to 3 mg ciprofloxacin base), 1 mg dexamethasone, USP, and 0.1 mg benzalkonium chloride as a preservative. The inactive ingredients are acetic acid, boric acid, edetate disodium (as dihydrate), hydroxyethyl cellulose, sodium acetate trihydrate, sodium chloride, tyloxapol and water for injection, USP. Sodium hydroxide or hydrochloric acid may be added for adjustment of pH.
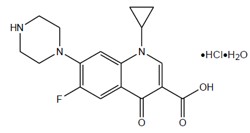
Ciprofloxacin, a quinolone antimicrobial is available as the monohydrochloride monohydrate salt of 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline carboxylic acid. The molecular formula is C17H18FN3O3·HCl·H2O. The molecular weight is 385.82 g/mol and the structural formula is:
Figure 1: Structure of Ciprofloxacin
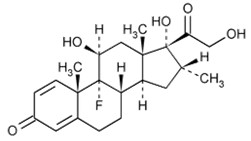
Dexamethasone USP, (11β,16α)-9-fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione, is a corticosteroid. The molecular formula is C22H29FO5. The molecular weight is 392.46 g/mol and the structural formula is:
Figure 2: Structure of Dexamethasone
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ciprofloxacin is a fluoroquinolone antibacterial [see Microbiology (12.4)].
Dexamethasone, a corticosteroid, has been shown to suppress inflammation by inhibiting multiple inflammatory cytokines resulting in decreased edema, fibrin deposition, capillary leakage and migration of inflammatory cells.
12.3 Pharmacokinetics
Following a single bilateral 4-drop (total dose = 0.28 mL, 0.84 mg ciprofloxacin, 0.28 mg dexamethasone) topical otic dose of ciprofloxacin and dexamethasone otic suspension to pediatric patients, measurable plasma concentrations of ciprofloxacin and dexamethasone were observed at 6 hours following administration in 2 of 9 patients and 5 of 9 patients, respectively.
Mean ± SD peak plasma concentrations of ciprofloxacin were 1.39 ± 0.880 ng/mL (n = 9). Peak plasma concentrations ranged from 0.543 ng/mL to 3.45 ng/mL and were on average approximately 0.1% of peak plasma concentrations achieved with an oral dose of 250 mg. Peak plasma concentrations of ciprofloxacin were observed within 15 minutes to 2 hours post dose application.
Mean ± SD peak plasma concentrations of dexamethasone were 1.14 ± 1.54 ng/mL (n = 9). Peak plasma concentrations ranged from 0.135 ng/mL to 5.10 ng/mL and were on average approximately 14% of peak concentrations reported in the literature following an oral 0.5 mg tablet dose. Peak plasma concentrations of dexamethasone were observed within 15 minutes to 2 hours post dose application.
Dexamethasone has been added to aid in the resolution of the inflammatory response accompanying bacterial infection.
12.4 Microbiology
Mechanism of Action
The bactericidal action of ciprofloxacin results from interference with the enzyme, DNA gyrase, which is needed for the synthesis of bacterial DNA.
Resistance
Cross-resistance has been observed between ciprofloxacin and other fluoroquinolones. There is generally no cross-resistance between ciprofloxacin and other classes of anti-bacterial agents, such as beta-lactams or aminoglycosides.
Antimicrobial Activity
Ciprofloxacin has been shown to be active against most isolates of the following microorganisms, both in vitro and clinically in otic infections [see Indications and Usage (1)].
Aerobic Bacteria
Gram-positive Bacteria
-
Staphylococcus aureus
Gram-negative Bacteria
- Pseudomonas aeruginosa
-
Staphylococcus aureus
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term carcinogenicity studies in mice and rats have been completed for ciprofloxacin. After daily oral doses of 750 mg/kg (mice) and 250 mg/kg (rats) were administered for up to 2 years, there was no evidence that ciprofloxacin had any carcinogenic or tumorigenic effects in these species. No long-term studies of ciprofloxacin and dexamethasone otic suspension have been performed to evaluate carcinogenic potential. Long-term studies have not been performed to evaluate the carcinogenic potential of topical otic dexamethasone.
Mutagenesis
Eight in vitro mutagenicity tests have been conducted with ciprofloxacin, and the test results are listed below:
- Salmonella/Microsome Test (Negative)
- E. coli DNA Repair Assay (Negative)
- Mouse Lymphoma Cell Forward Mutation Assay (Positive)
- Chinese Hamster V79 Cell HGPRT Test (Negative)
- Syrian Hamster Embryo Cell Transformation Assay (Negative)
- Saccharomyces cerevisiae Point Mutation Assay (Negative)
- Saccharomyces cerevisiae Mitotic Crossover and Gene Conversion Assay (Negative)
- Rat Hepatocyte DNA Repair Assay (Positive)
Thus, 2 of the 8 tests were positive, but results of the following 3 in vivo test systems gave negative results:
- Rat Hepatocyte DNA Repair Assay
- Micronucleus Test (Mice)
- Dominant Lethal Test (Mice)
Dexamethasone has been tested for in vitro and in vivo genotoxic potential and shown to be positive in the following assays: chromosomal aberrations, sister-chromatid exchange in human lymphocytes, and micronuclei and sister-chromatid exchanges in mouse bone marrow. However, the Ames/Salmonella assay, both with and without S9 mix, did not show any increase in His+ revertants.
Impairment of Fertility
Fertility studies performed in male and female rats at oral doses of ciprofloxacin up to 100 mg/kg (approximately 482 times the ROHD of ciprofloxacin based on BSA) revealed no evidence of impairment. Male rats received oral ciprofloxacin for 10 weeks prior to mating and females were dosed for 3 weeks prior to mating through GD 7.
The effect of dexamethasone on fertility has not been investigated following topical otic application. However, the lowest toxic dose of dexamethasone identified following topical dermal application was 1.802 mg/kg in a 26-week study in male rats and resulted in changes to the testes, epididymis, sperm duct, prostate, seminal vesicle, Cowper's gland, and accessory glands. The relevance of this study for short-term topical otic use is unknown.
-
14 CLINICAL STUDIES
In 2 randomized multicenter, controlled clinical trials, ciprofloxacin and dexamethasone otic suspension dosed 2 times per day for 7 days demonstrated clinical cures in 87% and 94% of per protocol evaluable AOE patients, respectively, compared to 84% and 89%, respectively, for otic suspension containing neomycin 0.35%, polymyxin B 10,000 units/mL, and hydrocortisone 1.0% (neo/poly/HC). Among culture-positive patients clinical cures were 86% and 92% for ciprofloxacin and dexamethasone otic suspension compared to 84% and 89%, respectively, for neo/poly/HC. Microbiological eradication rates for these patients in the same clinical trials were 86% and 92% for ciprofloxacin and dexamethasone otic suspension compared to 85% and 85%, respectively, for neo/poly/HC.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Ciprofloxacin 0.3% and Dexamethasone 0.1% Otic Suspension, USP, is a sterile white to off white homogenous suspension supplied as 7.5 mL fill in a system consisting of a 10 mL LDPE natural bottle, 30 µL LDPE natural nozzle and a HDPE white opaque screw-on cap with tamper evident ring for tamper evident feature.
It is available as follows:
7.5 mL in a 10 mL Container: NDC: 53746-831-53
Storage
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Avoid freezing. Protect from light.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
For Otic Use Only
Advise patients that ciprofloxacin and dexamethasone otic suspension is for otic use (ears) only. This product must not be used in the eye [see Dosage and Administration (2.2)].
Administration Instructions
Instruct patients to warm the bottle in their hand for one to two minutes prior to use and shake well immediately before using [see Dosage and Administration (2.1, 2.2)].
Allergic Reactions
Advise patients to discontinue use immediately and contact their physician, if rash or allergic reaction occurs [see Warnings and Precautions (5.1)].
Avoid Contamination of the Product
Advise patients to avoid contaminating the tip with material from the ear, fingers, or other sources [see Instructions for Use].
Duration of Use
Advise patients that it is very important to use the eardrops for as long as their doctor has instructed, even if the symptoms improve [see Patient Information].
Protect from Light
Advise patients to protect the product from light [see How Supplied/Storage and Handling (16)].
Unused Product
Advise patients to discard unused portion after therapy is completed [see Dosage and Administration (2.2)].
Manufactured by:
Amneal Pharmaceuticals Private Limited
Parenteral Unit
Ahmedabad 382213, INDIADistributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807Rev. 10-2022-00
-
PATIENT INFORMATION
Ciprofloxacin (sip״ roe flox׳ a sin) and Dexamethasone (dex״ a meth׳ a sone)
Otic Suspension, USP
What is ciprofloxacin and dexamethasone otic suspension?
Ciprofloxacin and dexamethasone otic suspension is a prescription medicine used in the ear only (otic use) that contains 2 medicines, a quinolone antibiotic medicine called ciprofloxacin and a corticosteroid medicine called dexamethasone. Ciprofloxacin and dexamethasone otic suspension is used in adults and children 6 months of age or older to treat certain types of infections caused by certain germs called bacteria. These bacterial infections include:
- outer ear canal infection (known as acute otitis externa or AOE)
It is not known if ciprofloxacin and dexamethasone otic suspension is safe and effective in children under 6 months of age.
Who should not use ciprofloxacin and dexamethasone otic suspension?
Do not use ciprofloxacin and dexamethasone otic suspension if you:
- are allergic to ciprofloxacin, quinolones, or any of the ingredients in ciprofloxacin and dexamethasone otic suspension. See the end of this Patient Information leaflet for a complete list of ingredients in ciprofloxacin and dexamethasone otic suspension.
- have an outer ear canal infection caused by certain viruses, including the herpes simplex virus.
- have an ear infection caused by a fungus.
What should I tell my doctor before using ciprofloxacin and dexamethasone otic suspension?
Before using ciprofloxacin and dexamethasone otic suspension, tell your doctor about all of your medical conditions, including if you:
- are pregnant or plan to become pregnant. It is not known if ciprofloxacin and dexamethasone otic suspension will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if ciprofloxacin and dexamethasone passes into your breast milk. Talk to your doctor about the best way to feed your baby during treatment with ciprofloxacin and dexamethasone otic suspension.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use ciprofloxacin and dexamethasone otic suspension?
- Read the detailed Instructions for Use that come with ciprofloxacin and dexamethasone otic suspension.
- Use ciprofloxacin and dexamethasone otic suspension exactly as your doctor tells you to.
- Ciprofloxacin and dexamethasone otic suspension is for use in the ear only (otic use). Do not use ciprofloxacin and dexamethasone otic suspension in the eye or inject ciprofloxacin and dexamethasone otic suspension.
- Apply 4 drops of ciprofloxacin and dexamethasone otic suspension into the affected ear 2 times a day for 7 days.
- Do not stop using ciprofloxacin and dexamethasone otic suspension unless your doctor tells you to, even if your symptoms improve.
If your symptoms do not improve after 7 days of treatment with ciprofloxacin and dexamethasone otic suspension, call your doctor.
- Call your doctor right away if:
- you have fluid that continues to drain from your ear (otorrhea) after you have finished your treatment with ciprofloxacin and dexamethasone otic suspension
- you have fluid that drains from your ear 2 or more times within 6 months after you stop treatment with ciprofloxacin and dexamethasone otic suspension
What are the possible side effects of ciprofloxacin and dexamethasone otic suspension?
Ciprofloxacin and dexamethasone otic suspension may cause serious side effects, including:
-
allergic reactions. Stop using ciprofloxacin and dexamethasone otic suspension and call your doctor or go to the nearest emergency room if you have any of the following signs or symptoms of an allergic reaction:
- hives (urticaria)
- swelling of your face, lips, mouth, or tongue
- rash
- itching
- trouble breathing
- dizziness, fast heartbeat, or pounding in your chest
The most common side effects of ciprofloxacin and dexamethasone otic suspension include:
- ear discomfort
- ear pain
- ear itching (pruritus)
These are not all the possible side effects of ciprofloxacin and dexamethasone otic suspension.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ciprofloxacin and dexamethasone otic suspension?
- Store ciprofloxacin and dexamethasone otic suspension at room temperature at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F).
- Do not freeze ciprofloxacin and dexamethasone otic suspension.
- Keep ciprofloxacin and dexamethasone otic suspension out of light.
Keep ciprofloxacin and dexamethasone otic suspension and all medicines out of the reach of children.
General information about the safe and effective use of ciprofloxacin and dexamethasone otic suspension.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ciprofloxacin and dexamethasone otic suspension for a condition for which it was not prescribed.
Do not give ciprofloxacin and dexamethasone otic suspension to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about ciprofloxacin and dexamethasone otic suspension that is written for health professionals.
What are the ingredients in ciprofloxacin and dexamethasone otic suspension?
Active ingredients: Ciprofloxacin hydrochloride, USP and Dexamethasone, USPInactive ingredients: Benzalkonium chloride as a preservative, acetic acid, boric acid, edetate disodium (as dihydrate), hydroxyethyl cellulose, sodium acetate trihydrate, sodium chloride, tyloxapol and water for injection, USP. Sodium hydroxide or hydrochloric acid may be added for adjustment of pH.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Amneal Pharmaceuticals Private Limited
Parenteral Unit
Ahmedabad 382213, INDIA
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 10-2022-00 -
INSTRUCTIONS
FOR USE
Ciprofloxacin (sip״ roe flox׳ a sin) and Dexamethasone (dex״ a meth׳ a sone) Otic Suspension, USP
This “Instructions for Use” contains information on how to use ciprofloxacin and dexamethasone otic suspension.Important Information You Need to Know Before Using Ciprofloxacin and Dexamethasone Otic Suspension. Read this Instructions for Use that comes with ciprofloxacin and dexamethasone otic suspension before you start using it and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or treatment.
- Use ciprofloxacin and dexamethasone otic suspension exactly as your doctor tells you to use it.
- Ciprofloxacin and dexamethasone otic suspension is for use in the ear only (otic use). Do not inject ciprofloxacin and dexamethasone otic suspension or use ciprofloxacin and dexamethasone otic suspension in the eye.
- Shake ciprofloxacin and dexamethasone otic suspension well before each use.
- Do not touch your ear, fingers, or other surfaces with the tip of the ciprofloxacin and dexamethasone otic suspension bottle. You may get bacteria on the tip of the bottle that can cause you to get another infection.
How should I use ciprofloxacin and dexamethasone otic suspension? Step 1. Wash your hands with soap and water (see Figure A).
Figure A
Step 2. Warm the bottle of ciprofloxacin and dexamethasone otic suspension by rolling the bottle between your hands for 1 to 2 minutes (see Figure B). Shake the bottle of ciprofloxacin and dexamethasone otic suspension well.
Figure B
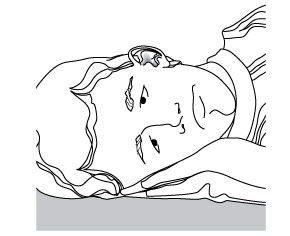
Step 3. Remove the ciprofloxacin and dexamethasone otic suspension cap. Put the cap in a clean and dry area. Do not let the tip of the bottle touch your ear, fingers or other surfaces. Step 4. Lie down on your side so that the affected ear faces upward (see Figure C).
Figure C
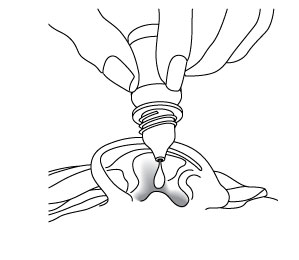
Step 5. Hold the bottle of ciprofloxacin and dexamethasone otic suspension between your thumb and index finger (see Figure D). Place the tip of the bottle close to your ear. Be careful not to touch your fingers or ear with the tip of the bottle.
Figure D
Step 6. Gently squeeze the bottle and let 4 drops of ciprofloxacin and dexamethasone otic suspension fall into the affected ear. If a drop misses your ear, follow the instructions in Step 5 again. Step 7. Stay on your side with the affected ear facing upward (see Figure C).
It is important that you follow the instructions below for your specific ear infection, to allow ciprofloxacin and dexamethasone otic suspension to enter the affected part of your ear.
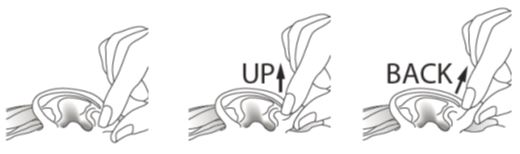
Step 8. If you use ciprofloxacin and dexamethasone otic suspension to treat an outer ear canal infection:
- Gently pull the outer ear lobe upward and backward (see Figure F). This will allow the drops of ciprofloxacin and dexamethasone otic suspension to enter your ear canal.
- Remain on your side with the affected ear facing upward (see Figure C) for 1 minute.
Figure F
Step 9. If your doctor has told you to use ciprofloxacin and dexamethasone otic suspension in both ears, repeat steps 5-8 for your other ear. Step 10. Put the cap back on the bottle and close it tightly. Step 11. After you have used all of your ciprofloxacin and dexamethasone otic suspension doses, there may be some ciprofloxacin and dexamethasone otic suspension left in the bottle. Throw the bottle away. How should I store ciprofloxacin and dexamethasone otic suspension?
- Store ciprofloxacin and dexamethasone otic suspension at room temperature at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F).
- Do not freeze ciprofloxacin and dexamethasone otic suspension.
- Keep ciprofloxacin and dexamethasone otic suspension out of light.
Keep ciprofloxacin and dexamethasone otic suspension and all medicines out of the reach of children.
If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for more information about ciprofloxacin and dexamethasone otic suspension that is written for health professionals.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:Amneal Pharmaceuticals Private Limited
Parenteral Unit
Ahmedabad 382213, INDIA
Distributed by:Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 10-2022-00 -
PRINCIPAL DISPLAY PANEL
NDC: 53746-831-53
Ciprofloxacin and Dexamethasone Otic Suspension, USP
0.3%/0.1% (7.5 mL)
Rx only
Container Label
Amneal Pharmaceuticals LLC

NDC: 53746-831-53
Ciprofloxacin and Dexamethasone Otic Suspension, USP
0.3%/0.1% (7.5 mL)
Rx only
Carton Label
Amneal Pharmaceuticals LLC
-
INGREDIENTS AND APPEARANCE
CIPROFLOXACIN AND DEXAMETHASONE
ciprofloxacin and dexamethasone suspension/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 53746-831 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CIPROFLOXACIN HYDROCHLORIDE (UNII: 4BA73M5E37) (CIPROFLOXACIN - UNII:5E8K9I0O4U) CIPROFLOXACIN 3 mg in 1 mL DEXAMETHASONE (UNII: 7S5I7G3JQL) (DEXAMETHASONE - UNII:7S5I7G3JQL) DEXAMETHASONE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BORIC ACID (UNII: R57ZHV85D4) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROCHLORIC ACID (UNII: QTT17582CB) HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) SODIUM ACETATE (UNII: 4550K0SC9B) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) TYLOXAPOL (UNII: Y27PUL9H56) WATER (UNII: 059QF0KO0R) Product Characteristics Color white (white to off white homogenous suspension) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 53746-831-53 1 in 1 CARTON 03/26/2024 1 7.5 mL in 1 BOTTLE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216501 03/26/2024 Labeler - Amneal Pharmaceuticals of New York LLC (123797875) Establishment Name Address ID/FEI Business Operations Amneal Pharmaceuticals Private Limited 860156658 analysis(53746-831) , manufacture(53746-831) , pack(53746-831) , sterilize(53746-831)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.