TOBRAMYCIN by Lifestar Pharma LLC / Mankind Pharma Limited TOBRAMYCIN solution
TOBRAMYCIN by
Drug Labeling and Warnings
TOBRAMYCIN by is a Prescription medication manufactured, distributed, or labeled by Lifestar Pharma LLC, Mankind Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TOBRAMYCIN INHALATION SOLUTION safely and effectively. See full prescribing information for TOBRAMYCIN INHALATION SOLUTION.
TOBRAMYCIN inhalation solution, for oral inhalation use
Initial U.S. Approval: 1975RECENT MAJOR CHANGES
Warnings and Precautions, Ototoxicity (5.2) 2/2023
INDICATIONS AND USAGE
Tobramycin inhalation solution is an aminoglycoside antibacterial indicated for the management of cystic fibrosis in adults and pediatric patients 6 years of age and older with Pseudomonas aeruginosa. (1)
Safety and efficacy have not been demonstrated in patients under the age of 6 years, patients with forced expiratory volume in 1 second (FEV1) <25% or >75% predicted, or patients colonized with Burkholderia cepacia. (1)
DOSAGE AND ADMINISTRATION
- For oral inhalation only. (2.1)
- The recommended dosage for adults and pediatric patients 6 years of age and older is one single-dose ampoule (300 mg) twice daily by oral inhalation in alternating periods of 28 days on drug, followed by 28 days off drug. (2.1)
- Dosage is not adjusted by weight. (2.1)
- Take doses as close to 12 hours apart as possible; but not less than 6 hours apart. (2.1)
- Administer each 300 mg dose by inhalation using a hand-held PARI LC PLUS Reusable Nebulizer with a DeVilbiss Pulmo-Aide compressor. (2.2)
DOSAGE FORMS AND STRENGTHS
Inhalation Solution: 300 mg per 5 mL solution in a single-dose ampoule (3) (3)
CONTRAINDICATIONS
Known hypersensitivity to any aminoglycoside (4)
WARNINGS AND PRECAUTIONS
- Bronchospasm: Can occur with inhalation of tobramycin inhalation solution. Treat as medically appropriate, if it occurs. (5.1)
- Ototoxicity: Tinnitus and hearing loss have been reported in patients receiving tobramycin inhalation solution. If noted, manage as medically appropriate, including potentially discontinuing tobramycin inhalation solution. (5.2)
- Nephrotoxicity: Has been associated with aminoglycosides as a class. If nephrotoxicity develops, manage the patient as medically appropriate, including potentially discontinuing tobramycin inhalation solution. (5.3)
- Neuromuscular Disorders: Aminoglycosides may aggravate muscle weakness because of a potential curare‐like effect on neuromuscular function. If neuromuscular blockade occurs, it may be reversed by the administration of calcium salts but mechanical assistance may be necessary. (5.4)
- Embryo-fetal Toxicity: Aminoglycosides can cause fetal harm (5.5, 8.1)
ADVERSE REACTIONS
Most common adverse reactions (incidence >5%) are increased cough, pharyngitis, increased sputum, dyspnea, hemoptysis, decreased lung function, voice alteration, taste perversion and rash. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Lifestar Pharma LLC at 1-888-995-4337 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Concurrent and/or sequential use of tobramycin inhalation solution with other drugs with neurotoxic, nephrotoxic, or ototoxic potential should be avoided (7.1)
- Concomitant administration with ethacrynic acid, furosemide, urea, or intravenous mannitol is not recommended due to possible enhancement of aminoglycoside toxicity (7.2).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Bronchospasm
5.2 Ototoxicity
5.3 Nephrotoxicity
5.4 Neuromuscular Disorders
5.5 Embryo-fetal Toxicity
5.6 Concomitant Use of Systemic Aminoglycosides
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs with Neurotoxic, Nephrotoxic or Ototoxic Potential
7.2 Diuretics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Tobramycin inhalation solution is indicated for the management of cystic fibrosis in adults and pediatric patients 6 years of age and older with Pseudomonas aeruginosa.
Safety and efficacy have not been demonstrated in patients under the age of 6 years, patients with forced expiratory volume in 1 second (FEV1) <25% or >75% predicted, or patients colonized with Burkholderia cepacia [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
Tobramycin inhalation solution is for oral inhalation only [see Dosage and Administration (2.2)]. The recommended dosage of tobramycin inhalation solution for both adults and pediatric patients 6 years of age and older is one single-dose ampoule (300 mg) administered twice daily for 28 days. Dosage is not adjusted by weight. All patients should be administered 300 mg twice daily.
Tobramycin inhalation solution is administered twice daily in alternating periods of 28 days. After 28 days of therapy, patients should stop tobramycin inhalation solution therapy for the next 28 days, and then resume therapy for the next 28 day on/28 day off cycle. The doses should be taken as close to 12 hours apart as possible; they should not be taken less than 6 hours apart.
If patients miss a dose, they should take it as soon as possible anytime up to 6 hours prior to their next scheduled dose. If less than 6 hours remain before the next dose, wait until their next scheduled dose.
2.2 Administration Instructions
Tobramycin inhalation solution is administered by oral inhalation over an approximately 15-minute period, using a hand-held PARI LC PLUS Reusable Nebulizer with a DeVilbiss Pulmo-Aide compressor. Tobramycin inhalation solution should not be diluted or mixed with dornase alfa or other medications in the nebulizer. Tobramycin inhalation solution is not for subcutaneous, intravenous or intrathecal administration.
Prior to administration of tobramycin inhalation solution, read the Patient Information/Instructions for Use for tobramycin inhalation solution for detailed information on how to use tobramycin inhalation solution and follow the manufacturer's instructions for use and care of the PARI LC PLUS Reusable Nebulizer and DeVilbiss Pulmo-Aide air compressor. Tobramycin inhalation solution is inhaled while the patient is sitting or standing upright and breathing normally through the mouthpiece of the nebulizer. Nose clips may help the patient breathe through the mouth.
Instruct patients on multiple therapies to take their medications, prior to inhaling tobramycin inhalation solution or as directed by their physician.
Tobramycin inhalation solution should not be used if it is cloudy, if there are particles in the solution, or if it has been stored at room temperature for more than 28 days.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Bronchospasm
Bronchospasm can occur with inhalation of tobramycin inhalation solution. In clinical studies with tobramycin inhalation solution, changes in FEV1 measured after the inhaled dose were similar in tobramycin inhalation solution and placebo groups. Bronchospasm that occurs during the use of tobramycin inhalation solution should be treated as medically appropriate.
5.2 Ototoxicity
Ototoxicity with use of tobramycin inhalation solution
Ototoxicity, manifested as both auditory and vestibular toxicity, has been reported with parenteral aminoglycosides.
Transient tinnitus occurred in eight tobramycin inhalation solution treated patients versus no placebo patients in the clinical studies. Tinnitus may be a sentinel symptom of ototoxicity, and therefore the onset of this symptom warrants further clinical investigation. Ototoxicity, as measured by complaints of hearing loss or by audiometric evaluations, did not occur with tobramycin inhalation solution therapy during clinical studies, however in postmarketing experience, patients receiving tobramycin inhalation solution have reported hearing loss. Vestibular toxicity may be manifested by vertigo, ataxia or dizziness. Patients with known or suspected auditory or vestibular dysfunction should be closely monitored when taking tobramycin inhalation solution. Monitoring might include obtaining audiometric evaluations and serum tobramycin levels. If ototoxicity is noted, the patient should be managed as medically appropriate, including potentially discontinuing tobramycin inhalation solution.
Risk of Ototoxicity Due to Mitochondrial DNA Variants
Cases of ototoxicity with aminoglycosides have been observed in patients with certain variants in the mitochondrially encoded 12S rRNA gene (MT-RNR1), particularly the m.1555A>G variant. Ototoxicity occurred in some patients even when their aminoglycoside serum levels were within the recommended range. Mitochondrial DNA variants are present in less than 1% of the general US population, and the proportion of the variant carriers who may develop ototoxicity as well as the severity of ototoxicity is unknown. In case of known maternal history of ototoxicity due to aminoglycoside use or a known mitochondrial DNA variant in the patient, consider alternative treatments other than aminoglycosides unless the increased risk of permanent hearing loss is outweighed by the severity of infection and lack of safe and effective alternative therapies.
5.3 Nephrotoxicity
Nephrotoxicity was not seen during clinical studies with tobramycin inhalation solution but has been associated with aminoglycosides as a class. Patients with known or suspected renal dysfunction or taking concomitant nephrotoxic drugs along with tobramycin inhalation solution should have serum concentrations of tobramycin and laboratory measurements of renal function obtained at the discretion of the treating physician. If nephrotoxicity develops, the patient should be managed as medically appropriate, including potentially discontinuing tobramycin inhalation solution.
5.4 Neuromuscular Disorders
Aminoglycosides, including tobramycin, may aggravate muscle weakness because of a potential curare-like effect on neuromuscular function. Neuromuscular blockade, respiratory failure, and prolonged respiratory paralysis may occur more commonly in patients with underlying neuromuscular disorders, such as myasthenia gravis or Parkinson's disease. Prolonged respiratory paralysis may also occur in patients receiving concomitant neuromuscular blocking agents. If neuromuscular blockade occurs, it may be reversed by the administration of calcium salts but mechanical assistance may be necessary.
5.5 Embryo-fetal Toxicity
Aminoglycosides can cause fetal harm when administered to a pregnant woman. Aminoglycosides cross the placenta, and streptomycin has been associated with several reports of total, irreversible, bilateral congenital deafness in pediatric patients exposed in utero. However, systemic absorption of tobramycin following inhaled administration is expected to be minimal [see Clinical Pharmacology (12.3)]. Patients who use tobramycin inhalation solution during pregnancy, or become pregnant while taking tobramycin inhalation solution should be apprised of the potential hazard to the fetus [see Use in Specific Populations (8.1)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Bronchospasm [see Warnings and Precautions (5.1)]
- Ototoxicity [see Warnings and Precautions (5.2)]
- Nephrotoxicity[see Warnings and Precautions (5.3)]
- Neuromuscular Disorders [see Warnings and Precautions (5.4)]
- Embryo-fetal Toxicity [see Warnings and Precautions (5.5)]
- Concomitant Use of Systemic Aminoglycosides [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Tobramycin inhalation solution was studied in two Phase 3 clinical studies involving 258 cystic fibrosis patients ranging in age from 6 to 48 years. Patients received tobramycin inhalation solution in alternating periods of 28 days on and 28 days off drug in addition to their standard cystic fibrosis therapy for a total of 24 weeks.
Table 1 lists the percent of patients with selected adverse reactions that occurred in >5% of tobramycin inhalation solution patients during the two Phase 3 studies.
Table 1: Percent of Patients With Selected Adverse Reactions Occurring in > 5% of Tobramycin Inhalation Solution Patients Adverse Reaction
Tobramycin Inhalation Solution
(n = 258)
%
Placebo
(n = 262)
%
Cough Increased
46.1
47.3
Pharyngitis
38.0
39.3
Sputum Increased
37.6
39.7
Dyspnea
33.7
38.5
Hemoptysis
19.4
23.7
Lung Function Decreased1
16.3
15.3
Voice Alteration
12.8
6.5
Taste Perversion
6.6
6.9
Rash
5.4
6.1
1 Includes reported decreases in pulmonary function tests or decreased lung volume on chest radiograph associated with intercurrent illness or study drug administration.
Selected adverse reactions that occurred in less than or equal to 5% of patients treated with tobramycin inhalation solution:
Ear and Labyrinth Disorders: Tinnitus
Musculoskeletal and Connective Tissue Disorders: Myalgia
Infections and Infestations: Laryngitis
Voice Alteration and Tinnitus
Voice alteration and tinnitus were the only adverse reactions reported by significantly more tobramycin inhalation solution-treated patients. Thirty- three patients (13%) treated with tobramycin inhalation solution complained of voice alteration compared to 17 (7%) placebo patients. Voice alteration was more common in the on-drug periods.
Eight patients from the tobramycin inhalation solution group (3%) reported tinnitus compared to no placebo patients. All episodes were transient, resolved without discontinuation of the tobramycin inhalation solution treatment regimen, and were not associated with loss of hearing in audiograms. Tinnitus is one of the sentinel symptoms of cochlear toxicity, and patients with this symptom should be carefully monitored for high frequency hearing loss. The numbers of patients reporting vestibular adverse experiences such as dizziness were similar in the tobramycin inhalation solution and placebo groups.
Changes in Serum Creatinine
Nine (3%) patients in the tobramycin inhalation solution group and nine (3%) patients in the placebo group had increases in serum creatinine of at least 50% over baseline. In all nine patients in the tobramycin inhalation solution group, creatinine decreased at the next visit.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of tobramycin inhalation solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Ear and Labyrinth Disorders
Hearing loss: Some of these reports occurred in patients with previous or concomitant treatment with systemic aminoglycosides. Patients with hearing loss frequently reported tinnitus. [see Warnings and Precautions (5.2)]
Skin and Subcutaneous Tissue Disorders
Hypersensitivity, pruritus, urticaria, rash
Nervous System Disorders
Aphonia, dysgeusia
Respiratory, Thoracic, and Mediastinal Disorders
Bronchospasm [see Warnings and Precautions (5.1)] oropharyngeal pain
Metabolism and Nutrition Disorders
Decreased appetite
-
7 DRUG INTERACTIONS
7.1 Drugs with Neurotoxic, Nephrotoxic or Ototoxic Potential
Concurrent and/or sequential use of tobramycin inhalation solution with other drugs with neurotoxic, nephrotoxic, or ototoxic potential should be avoided.
7.2 Diuretics
Some diuretics can enhance aminoglycoside toxicity by altering aminoglycoside concentrations in serum and tissue. Tobramycin inhalation solution should not be administered concomitantly with ethacrynic acid, furosemide, urea, or intravenous mannitol. The interaction between inhaled mannitol and tobramycin inhalation solution has not been evaluated.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Aminoglycosides can cause fetal harm. Published literature reports that use of streptomycin, an aminoglycoside, can cause total, irreversible, bilateral congenital deafness when administered to a pregnant woman [see Warnings and Precautions (5.5)]. Although there are no available data on tobramycin inhalation solution use in pregnant women to inform a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes, systemic absorption of tobramycin following inhaled administration is expected to be minimal [see Clinical Pharmacology (12.3)]. There are risks to the mother associated with cystic fibrosis in pregnancy (see Clinical Considerations). In animal reproduction studies with subcutaneous administration of tobramycin in pregnant rats and rabbits during organogenesis there were no adverse developmental outcomes; however, ototoxicity was not evaluated in the offspring from these studies (see Data). Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Cystic fibrosis may increase the risk for preterm delivery.
Data
Animal Data
No reproductive toxicity studies have been conducted with tobramycin inhalation solution (tobramycin administered by inhalation). However, subcutaneous administration of tobramycin at doses of up to 100 (rat) or 20 (rabbit) mg/kg/day during organogenesis was not associated with adverse developmental outcomes. Doses of tobramycin ≥40 mg/kg/day were severely maternally toxic to rabbits and precluded the evaluation of adverse developmental outcomes. Ototoxicity was not evaluated in offspring during non-clinical reproductive toxicity studies with tobramycin.
8.2 Lactation
There are no data on the presence of tobramycin in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. Limited published data on other formulations of tobramycin in lactating women indicate that tobramycin is present in human milk. However, systemic absorption of tobramycin following inhaled administration is expected to be minimal [see Clinical Pharmacology (12.3)]. Tobramycin may cause alteration in the intestinal flora of the breastfeeding infant (see Clinical Considerations). The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for tobramycin inhalation solution and any potential adverse effects on the breastfed infant from tobramycin inhalation solution or from the underlying maternal condition.
Clinical Considerations
Tobramycin may cause intestinal flora alteration. Advise a woman to monitor the breastfed infant for loose or bloody stools and candidiasis (thrush, diaper rash).
8.4 Pediatric Use
The safety and efficacy of tobramycin inhalation solution in pediatric patients under 6 years of age has not been established. The use of tobramycin inhalation solution is not indicated in children <6 years of age [see Indications and Usage (1) and Dosage and Administration (2)].
8.5 Geriatric Use
Clinical studies of tobramycin inhalation solution did not include patients aged 65 years and over. Tobramycin is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function [see Warnings and Precautions (5.3)].
-
10 OVERDOSAGE
Signs and symptoms of acute toxicity from overdosage of intravenous (IV) tobramycin might include dizziness, tinnitus, vertigo, loss of high-tone hearing acuity, respiratory failure, neuromuscular blockade, and renal impairment. Administration by inhalation results in low systemic bioavailability of tobramycin. Tobramycin is not significantly absorbed following oral administration. Tobramycin serum concentrations may be helpful in monitoring overdosage.
Acute toxicity should be treated with immediate withdrawal of tobramycin inhalation solution, and baseline tests of renal function should be undertaken.
In all cases of suspected overdosage, physicians should contact the Regional Poison Control Center for information about effective treatment. In the case of any overdosage, the possibility of drug interactions with alterations in drug disposition should be considered.
Hemodialysis may be helpful in removing tobramycin from the body.
-
11 DESCRIPTION
Tobramycin inhalation solution, USP is a tobramycin solution for inhalation. It is a sterile, clear, slightly yellow, non-pyrogenic, aqueous solution with the pH and salinity adjusted specifically for administration by a compressed air driven reusable nebulizer. The chemical formula for tobramycin is C18H37N5O9 and the molecular weight is 467.52 g/mol. Tobramycin is O-3-amino-3-deoxy-α-D-glucopyranosyl-(1→4)-O-[2,6-diamino-2,3,6- trideoxy-α-D-ribo-hexopyranosyl-(1→6)]-2-deoxy-L-streptamine. The structural formula for tobramycin is:
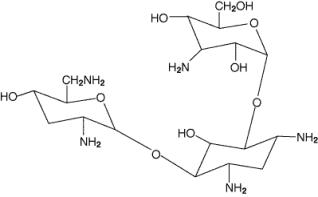
Each single-dose 5 mL ampoule contains 300 mg tobramycin and 11.25 mg sodium chloride in sterile water for injection. Sulfuric acid and sodium hydroxide are added to adjust the pH to 6.0. Nitrogen is used for sparging. All ingredients meet USP requirements. The formulation contains no preservatives.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Tobramycin inhalation solution contains tobramycin, a cationic polar molecule that does not readily cross epithelial membranes.(1) The bioavailability of tobramycin inhalation solution may vary because of individual differences in nebulizer performance and airway pathology.(2) Following administration of tobramycin inhalation solution, tobramycin remains concentrated primarily in the airways.
Serum Concentrations
The average serum concentration of tobramycin one hour after inhalation of a single 300-mg dose of tobramycin inhalation solution by cystic fibrosis patients was 0.95 mcg/mL. After 20 weeks of therapy on the tobramycin inhalation solution regimen, the average serum tobramycin concentration one hour after dosing was 1.05 mcg/mL.
Sputum Concentrations
Ten minutes after inhalation of the first 300-mg dose of tobramycin inhalation solution by cystic fibrosis patients, the average concentration of tobramycin was 1237 mcg/g (range 35 to 7417 mcg/g) in sputum. Tobramycin does not accumulate in sputum; after 20 weeks of therapy with the tobramycin inhalation solution regimen, the average concentration of tobramycin at ten minutes after inhalation was 1154 mcg/g (range 39 to 8085 mcg/g) in sputum. Two hours after inhalation, sputum concentrations declined to approximately 14% of tobramycin levels at ten minutes after inhalation.
Distribution
Following administration of tobramycin inhalation solution, tobramycin remains concentrated primarily in the airways. Binding of tobramycin to serum proteins is negligible.
Elimination
Metabolism
Tobramycin is not metabolized.
Excretion
The elimination half-life of tobramycin from serum is approximately 2 and 3 hours after intravenous (IV) administration and inhalation, respectively. Systemically absorbed tobramycin is primarily excreted unchanged in the urine principally by glomerular filtration. Unabsorbed tobramycin, following tobramycin inhalation solution administration, is probably eliminated primarily in expectorated sputum.
12.4 Microbiology
Tobramycin is an aminoglycoside antibacterial produced by Streptomyces tenebrarius.(1)It acts primarily by disrupting protein synthesis, leading to altered cell membrane permeability, progressive disruption of the cell envelope, and eventual cell death.(3)
Tobramycin has in vitro activity against gram-negative bacteria including Pseudomonas aeruginosa. It is bactericidal in vitro at concentrations equal to or slightly greater than the minimum inhibitory concentration (MIC).
Resistance
Treatment for 6 months with tobramycin inhalation solution in two clinical studies did not affect the susceptibility of the majority of P. aeruginosa isolates tested; however, increased minimum inhibitory concentrations (MICs) were noted in some patients. The clinical significance of this information has not been clearly established in the treatment of P. aeruginosa in cystic fibrosis patients [see Clinical Studies (14)].
Susceptibility Test Methods
Interpretive criteria for inhaled antibacterial products are not defined. The in vitro antimicrobial susceptibility test methods used for parenteral tobramycin therapy can be used to monitor the susceptibility of P. aeruginosa isolated from cystic fibrosis patients. If decreased susceptibility is noted, the results should be reported to the clinician.
Susceptibility breakpoints established for parenteral administration of tobramycin do not apply to aerosolized administration of tobramycin inhalation solution. The relationship between in vitro susceptibility test results and clinical outcome with tobramycin inhalation solution therapy is not clear.
A single sputum sample from a cystic fibrosis patient may contain multiple morphotypes of Pseudomonas aeruginosa and each morphotype may have a different level of in vitro susceptibility to tobramycin.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A two-year rat inhalation toxicology study to assess carcinogenic potential of tobramycin inhalation solution has been completed. Rats were exposed to tobramycin inhalation solution for up to 1.5 hours per day for 95 weeks. The clinical formulation of the drug was used for this carcinogenicity study. Serum levels of tobramycin of up to 35 mcg/mL were measured in rats, in contrast to the average 1 mcg/mL levels observed in cystic fibrosis patients in clinical trials. There was no drug-related increase in the incidence of any variety of tumor.
Additionally, tobramycin has been evaluated for genotoxicity in a battery of in vitro and in vivo tests. The Ames bacterial reversion test, conducted with 5 tester strains, failed to show a significant increase in revertants with or without metabolic activation in all strains. Tobramycin was negative in the mouse lymphoma forward mutation assay, did not induce chromosomal aberrations in Chinese hamster ovary cells, and was negative in the mouse micronucleus test.
Subcutaneous administration of up to 100 mg/kg of tobramycin did not affect mating behavior or cause impairment of fertility in male or female rats.
-
14 CLINICAL STUDIES
Two identically designed, double-blind, randomized, placebo-controlled, parallel group, 24-week clinical studies (Study 1 and Study 2) at a total of 69 cystic fibrosis centers in the United States were conducted in cystic fibrosis patients with P. aeruginosa. Subjects who were less than 6 years of age, had a baseline creatinine of >2 mg/dL, or had Burkholderia cepacia isolated from sputum were excluded.
All subjects had baseline FEV1 % predicted between 25% and 75%. In these clinical studies, 258 patients received tobramycin inhalation solution therapy on an outpatient basis (see Table 2) using a hand-held PARI LC PLUS Reusable Nebulizer with a DeVilbiss Pulmo-Aide compressor.
Table 2: Dosing Regimens in Clinical Studies Cycle 1
Cycle 2
Cycle 3
28 days
28 days
28 days
28 days
28 days
28 days
Tobramycin Inhalation Solution
regimen
n=258
Tobramycin Inhalation Solution
300 mg
twice daily
No drug
Tobramycin Inhalation Solution
300 mg
twice daily
No drug
Tobramycin Inhalation Solution
300 mg
twice daily
No drug
Placebo
regimen
n=262
placebo
twice daily
No drug
placebo
twice daily
No drug
placebo
twice daily
No drug
All patients received either tobramycin inhalation solution or placebo (saline with 1.25 mg quinine for flavoring) in addition to standard treatment recommended for cystic fibrosis patients, which included oral and parenteral antipseudomonal therapy, β2-agonists, cromolyn, inhaled steroids, and airway clearance techniques. In addition, approximately 77% of patients were concurrently treated with dornase alfa (PULMOZYME, Genentech).
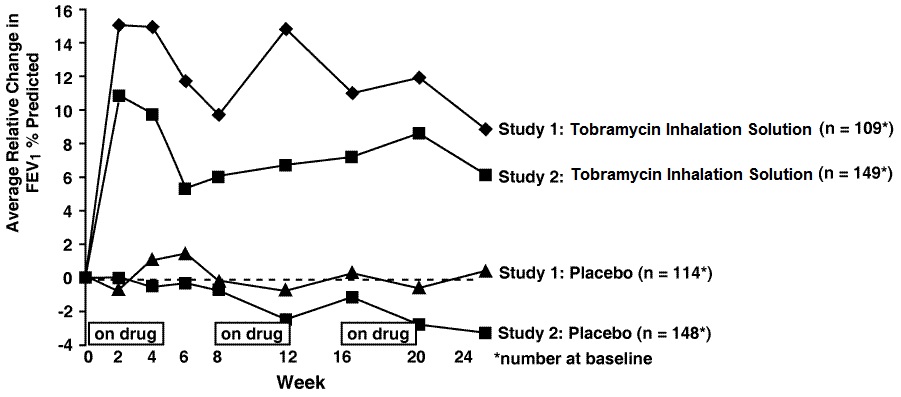
In each study, tobramycin inhalation solution -treated patients experienced significant improvement in pulmonary function. Improvement was demonstrated in the tobramycin inhalation solution group in Study 1 by an average increase in FEV1 % predicted of about 11% relative to baseline (Week 0) during 24 weeks compared to no average change in placebo patients. In Study 2, tobramycin inhalation solution-treated patients had an average increase of about 7% compared to an average decrease of about 1% in placebo patients. Figure 1 shows the average relative change in FEV1% predicted over 24 weeks for both studies.
Figure 1: Relative Change From Baseline in FEV1% Predicted
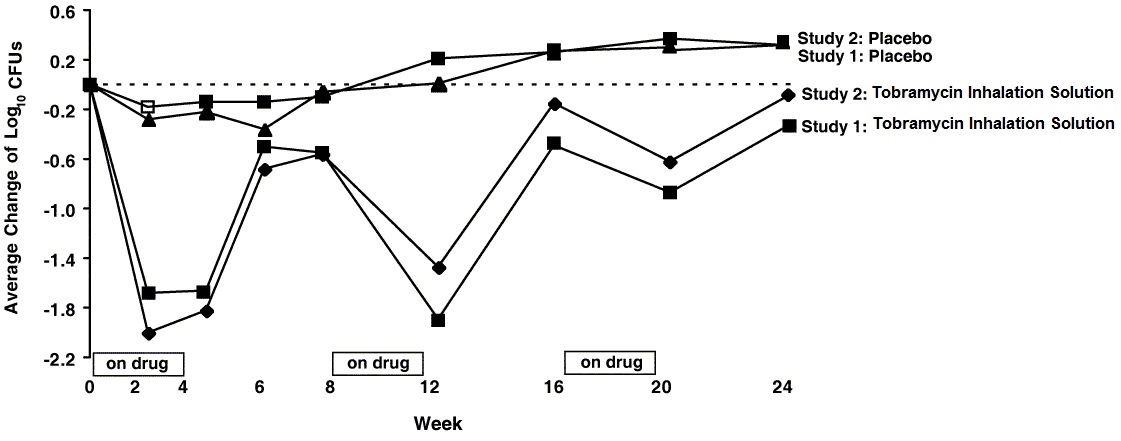
In each study, tobramycin inhalation solution therapy resulted in a significant reduction in the number of P. aeruginosa colony forming units (CFUs) in sputum during the on-drug periods. Sputum bacterial density returned to baseline during the off-drug periods. Reductions in sputum bacterial density were smaller in each successive cycle (see Figure 2).
Figure 2: Absolute Change From Baseline in Log10 CFUs
Patients treated with tobramycin inhalation solution were hospitalized for an average of 5.1 days compared to 8.1 days for placebo patients. Patients treated with tobramycin inhalation solution required an average of 9.6 days of parenteral antipseudomonal, antibacterial treatment compared to 14.1 days for placebo patients. During the 6 months of treatment, 40% of tobramycin inhalation solution patients and 53% of placebo patients were treated with parenteral antipseudomonal antibacterials.
The relationship between in vitro susceptibility test results and clinical outcome with tobramycin inhalation solution therapy is not clear. However, four tobramycin inhalation solution patients who began the clinical trial with P. aeruginosa isolates having MIC values ≥ 128 mcg/mL did not experience an improvement in FEV1 or a decrease in sputum bacterial density.
Treatment with tobramycin inhalation solution did not affect the susceptibility of the majority of P. aeruginosa isolates during the 6-month studies. However, some P. aeruginosa isolates did exhibit increased tobramycin MICs. The percentage of patients with P. aeruginosa isolates with tobramycin MICs ≥ 16 mcg/mL was 13% at the beginning, and 23% at the end of 6 months of the tobramycin inhalation solution regimen.
-
15 REFERENCES
- Neu HC. Tobramycin: an overview. [Review]. J Infect Dis 1976; Suppl 134:S3-19.
- Weber A, Smith A, Williams-Warren J et al. Nebulizer delivery of tobramycin to the lower respiratory tract. Pediatr Pulmonol 1994; 17 (5):331-9.
- Bryan LE. Aminoglycoside resistance. Bryan LE, Ed. Antimicrobial drug resistance. Orlando, FL: Academic Press, 1984: 241-77.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Tobramycin inhalation solution, USP is supplied as a sterile, clear, slightly yellow, non-pyrogenic, aqueous solution packaged in a 5 mL single-dose ampoule (300 mg tobramycin) for nebulization.
Tobramycin inhalation solution, USP 300 mg is available as follows:
5 mL single-dose ampoule (carton of 56) NDC: 70756-604-56
16.2 Storage and Handling
Tobramycin inhalation solution, USP should be stored under refrigeration at 2ºC–8ºC/36ºF–46ºF. Upon removal from the refrigerator, or if refrigeration is unavailable, tobramycin inhalation solution, USP pouches (opened or unopened) may be stored at room temperature (up to 25ºC/77ºF) for up to 28 days. Tobramycin inhalation solution, USP should not be used beyond the expiration date stamped on the ampoule when stored under refrigeration (2ºC– 8ºC/36ºF–46ºF) or beyond 28 days when stored at room temperature (25ºC/77ºF).
Tobramycin inhalation solution ampoules should not be exposed to intense light. The solution in the ampoule is slightly yellow, but may darken with age if not stored in the refrigerator; however, the color change does not indicate any change in the quality of the product as long as it is stored within the recommended storage conditions.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Difficulty Breathing:
Advise patients to inform their physicians if they experience shortness of breath or wheezing after administration of tobramycin inhalation solution. Tobramycin inhalation solution can cause a narrowing of the airway [see Warnings and Precautions (5.1)].
Hearing Loss:
Advise patients to inform their physician if they experience ringing in the ears, dizziness, or any changes in hearing because tobramycin inhalation solution has been associated with hearing loss [see Warnings and Precautions (5.2)].
Kidney Damage:
Advise patients to inform their physician if they have any history of kidney problems because tobramycin inhalation solution is in a class of drugs that have caused kidney damage [see Warnings and Precautions (5.3)].
Embryo-fetal Toxicity:
Advise pregnant women that aminoglycosides can cause irreversible congenital deafness when administered to a pregnant woman [see Warnings and Precautions (5.5) and Use in Specific Populations (8.1)].
Lactation:
Advise a woman to monitor their breastfed infants for diarrhea and/or bloody stools [see Use in Specific Populations (8.2)]
Manufactured for:
Lifestar Pharma LLC
1200 MacArthur Blvd.
Mahwah, NJ 07430 USA
Product of Hungary
Revised: 03/2023, H-01
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
Tobramycin Inhalation Solution (toe-brah-MYE-sin)
for oral inhalation use
What is Tobramycin Inhalation Solution ?
Tobramycin inhalation solution is a prescription medicine that is used to treat people with cystic fibrosis who have a bacterial infection called Pseudomonas aeruginosa. Tobramycin inhalation solution contains an antibacterial medicine called tobramycin (an aminoglycoside).
It is not known if tobramycin inhalation solution is safe and effective:
- in children under 6 years of age
- in people who have an FEV1 less than 25% or greater than 75% predicted
- in people who are colonized with a bacterium called Burkholderia cepacia
Do not take tobramycin inhalation solution if you are allergic to tobramycin, any of the ingredients in tobramycin inhalation solution, or to any other aminoglycoside antibacterial.
See the end of this Patient Information for a complete list of ingredients in tobramycin inhalation solution .
Before you take tobramycin inhalation solution , tell your healthcare provider about all of your medical conditions, including if you:
- have or have had hearing problems (including noises in your ears such as ringing or hissing), hearing loss, or your mother has had hearing problems after taking an aminoglycoside.
- have been told you have certain gene variants (a change in the gene) related to hearing abnormalities inherited from your mother.
- have dizziness
- have or have had kidney problems
- have or have had problems with muscle weakness such as myasthenia gravis or Parkinson's disease
- have or have had breathing problems such as wheezing, coughing, or chest tightness
- are pregnant or plan to become pregnant. Tobramycin inhalation solution is in a class of drugs that can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if tobramycin passes into your breast milk.
- are receiving aminoglycoside therapy by injection or through a vein (intravenous) while taking tobramycin inhalation solution. Your blood levels of tobramycin will be checked.
How should I take tobramycin inhalation solution ?
- See the step-by-step Instructions for Use about the right way to take your tobramycin inhalation solution.
- Take tobramycin inhalation solution exactly as your healthcare provider tells you. Do not change your dose or stop taking tobramycin inhalation solution unless your healthcare provider tells you to.
- The usual dose for adults and children over 6 years of age is:
- 1 single-dose ampule of tobramycin inhalation solution inhaled 2 times each day using a hand-held PARI LC PLUS™ Reusable Nebulizer and a DeVilbiss® Pulmo-Aide® air compressor.
- Each dose of tobramycin inhalation solution should be taken as close to 12 hours apart as possible.
- You should not take your dose less than 6 hours apart.
- Tobramycin inhalation solution is taken as a breathing treatment (inhalation) with a hand-held PARI LC PLUS Reusable Nebulizer with a DeVilbiss Pulmo-Aide compressor. Do not use any other nebulizer for your tobramycin inhalation solution treatment.
- Do not mix or dilute tobramycin inhalation solution with dornase alfa or other medicines in your nebulizer system.
- Each treatment should take about 15 minutes.
- Tobramycin inhalation solution should be inhaled while you are sitting or standing upright and breathing normally through the mouthpiece of the nebulizer. Nose clips may help you to breathe through your mouth.
- If you forget to take tobramycin inhalation solution and there are at least 6 hours to your next dose, take your dose as soon as you can. Otherwise, wait for your next dose. Do not double the dose to make up for the missed dose.
- After using tobramycin inhalation solution for 28 days, you should stop using it and wait 28 days. After you have stopped using tobramycin inhalation solution for 28 days, you should start using tobramycin inhalation solution again for 28 days. Complete the full 28-day course even if you are feeling better. It is important that you keep to the 28-day on, 28-day off cycle.
Using tobramycin inhalation solution with certain other medicines can cause serious side effects.
If you are using tobramycin inhalation solution, you should discuss with your healthcare provider if you should take:
- other medicines that may harm your nervous system, kidneys, or hearing
- "water pills" (diuretics) such as ethacrynic acid, furosemide, or intravenous mannitol
- urea
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
What are the possible side effects of tobramycin inhalation solution ?
Tobramycin inhalation solution may cause serious side effects, including:
- severe breathing problems (bronchospasm). Tell your healthcare provider right away if you get any of these symptoms of bronchospasm with using tobramycin inhalation solution:
- shortness of breath with wheezing
- coughing and chest tightness
- hearing loss or ringing in the ears (ototoxicity). Tell your healthcare provider right away if you have hearing loss or you hear noises in your ears such as ringing or hissing. Tell your healthcare provider if you develop vertigo, difficulty with balance or dizziness.
- worsening kidney problems (nephrotoxicity). Tobramycin inhalation solution is in a class of drugs which may cause worsening kidney problems, especially in people with known or suspected kidney problems. Your healthcare provider may do a blood test to check how your kidneys are working while you are using tobramycin inhalation solution.
- worsening muscle weakness (neuromuscular disorder). Tobramycin inhalation solution is in a class of drugs which can cause muscle weakness to get worse in people who already have problems with muscle weakness (myasthenia gravis or Parkinson's disease).
The most common side effects of tobramycin inhalation solution include:
- increased cough
- coughing up blood
- voice changes
- sore throat
- decreased lung function
- loss or change in taste
- increased sputum
- trouble breathing
- rash
These are not all of the possible side effects of tobramycin inhalation solution.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of tobramycin inhalation solution
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use tobramycin inhalation solution for a condition for which it was not prescribed. Do not give it to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for more information about tobramycin inhalation solution that is written for health professionals.
What are the ingredients in Tobramycin Inhalation Solution ?
Active ingredient: tobramycin
Inactive ingredients: sodium chloride in sterile water for injection, sulfuric acid, sodium hydroxide, and nitrogen
What is Pseudomonas aeruginosa?
It is a very common bacterium that infects the lungs of nearly everyone with cystic fibrosis at some time during their lives. Some people do not get this infection until later in their lives, while others get it very young. It is one of the most damaging bacteria for people with cystic fibrosis. If the infection is not properly managed, it will continue to damage your lungs causing further problems to your breathing.
Manufactured for:
Lifestar Pharma LLC
1200 MacArthur Blvd.
Mahwah, NJ 07430 USA
Product of Hungary
For more information, go to www.lifestarpharma.com or call Lifestar Pharma LLC at 1-888-995-4337.
This Patient Information has been approved by the U.S. Food and Drug Administration
Revised : 03/2023, H-01
Tobramycin
(toe-brah-MYE-sin)
Inhalation Solution
for oral inhalation use
Read this Instructions for Use before you start using tobramycin inhalation solution and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or treatment.
Tobramycin inhalation solution is made for inhalation using a PARI LC PLUS™ Reusable Nebulizer and a DeVilbiss® Pulmo-Aide® air compressor. Tobramycin inhalation solution can be taken at home, school, or at work. The following instructions tell you how to use the DeVilbiss Pulmo-Aide air compressor and PARI LC PLUS Reusable Nebulizer to administer tobramycin inhalation solution.
You will need the following supplies (See Figure A):
- 1 tobramycin inhalation solution plastic ampule (tobramycin inhalation solution is packaged with 4 ampules in each foil pouch)
- DeVilbiss Pulmo-Aide air compressor
- PARI LC PLUS Reusable Nebulizer
- Tubing to connect the nebulizer and compressor
- Clean paper or cloth towels
- Nose clips (optional)
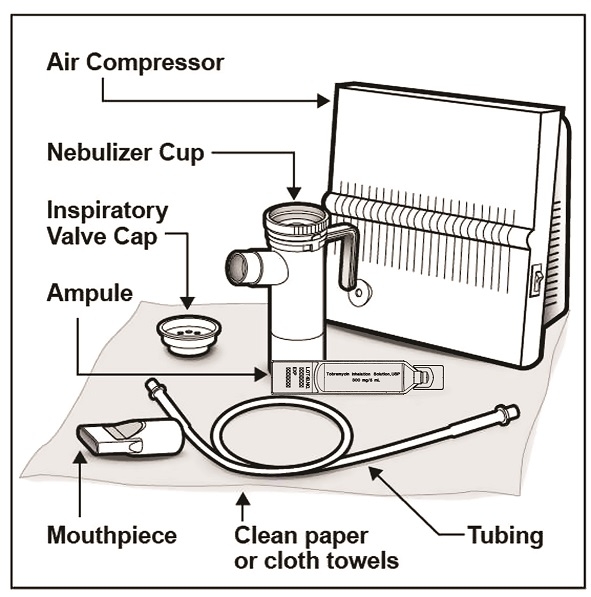
It is important that your nebulizer and compressor function properly before starting your tobramycin inhalation solution therapy. Note: Read the manufacturer care and use instructions for important information.
Prepare Your Tobramycin Inhalation Solution for Inhalation Therapy
Step 1: Wash your hands thoroughly with soap and water.
Step 2: Open the foil pouch.
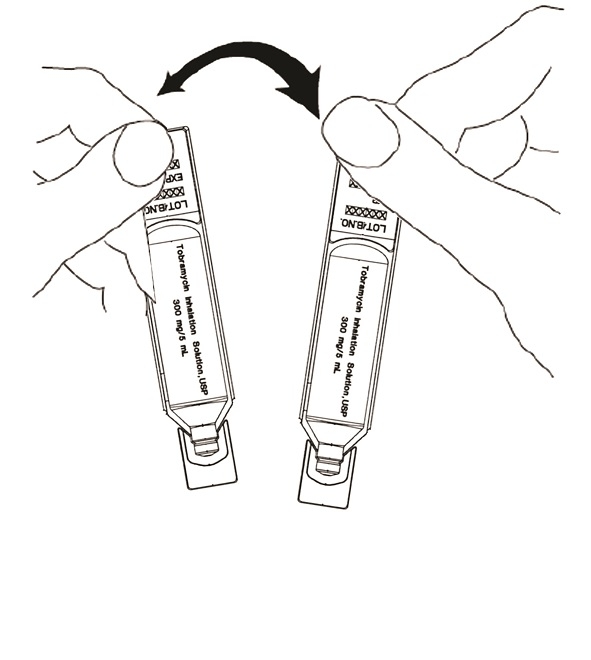
Step 3: Separate 1 tobramycin inhalation solution ampule by gently pulling apart at the bottom tabs (See Figure B). Place the remaining tobramycin inhalation solution ampules in the refrigerator.
(Figure B)
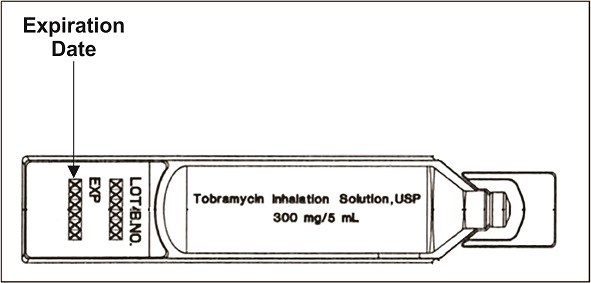
Step 4: Check the expiration date stamped on the tobramycin inhalation solution ampule (See Figure C). Do not use the tobramycin inhalation solution ampule if the expiration date has passed.
(Figure C)
Step 5: Check that the tobramycin inhalation solution ampule medicine is clear and does not have particles.
- Unrefrigerated tobramycin inhalation solution, which is normally slightly yellow, may darken with age. This color change does not mean there is any change in the quality of the medicine.
- Do not use the tobramycin inhalation solution ampule if the medicine is cloudy or has particles.
- Throw it away and get a new one.
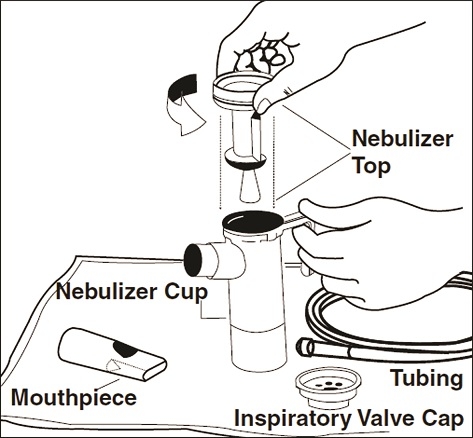
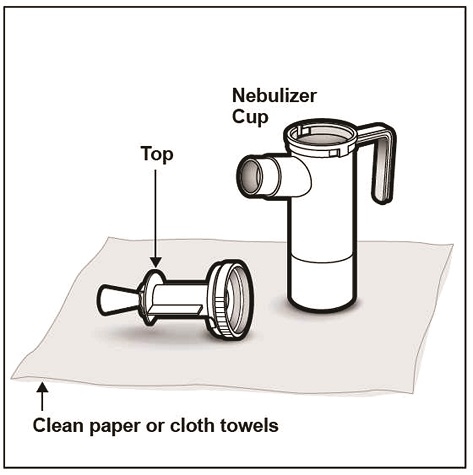
Step 6: Lay out the parts of a PARI LC PLUS Reusable Nebulizer package on a clean, dry paper or cloth towel. You should have the following parts (See Figure D):
- Nebulizer Top and Bottom (Nebulizer Cup) Assembly
- Inspiratory Valve Cap
- Mouthpiece with Valve
- Tubing
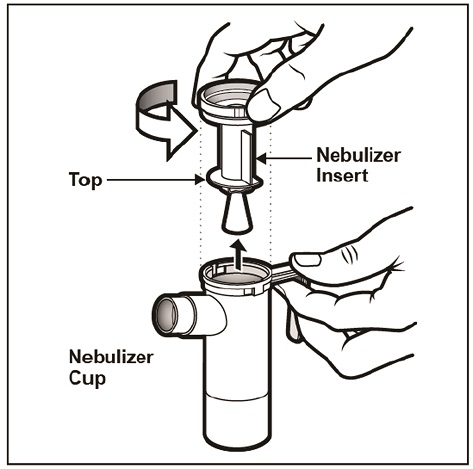
Step 7: Remove the Nebulizer Top from the Nebulizer Cup by twisting the Nebulizer Top counter-clockwise, and then lifting off (See Figure E).
(Figure E)
Step 8: Place the Nebulizer Top on the clean paper or cloth towel by standing the Nebulizer Cup upright on the towel (See Figure F).
(Figure F)
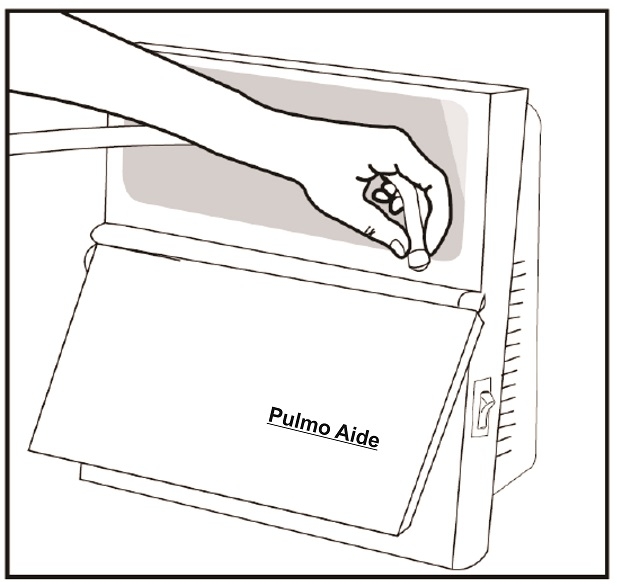
Step 9: Connect one end of the tubing to the compressor air outlet (See Figure G). The tubing should fit tightly.
(Figure G)
Step 10: Plug in your compressor to an electrical outlet (See Figure H).
(Figure H)
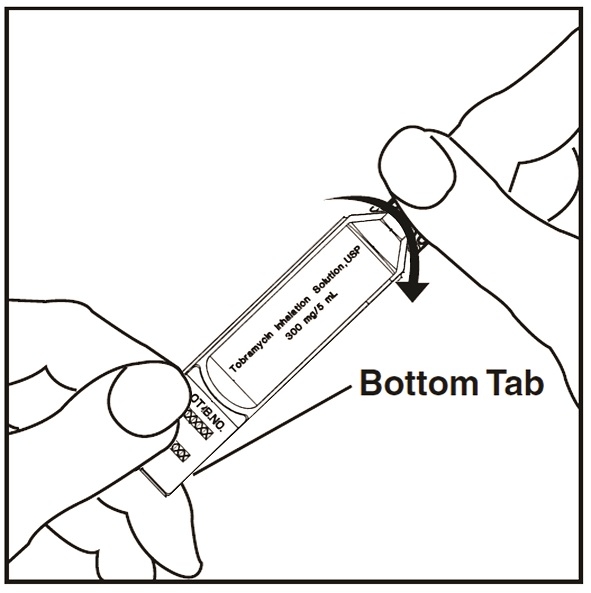
Step 11: Open the tobramycin inhalation solution ampule by holding the bottom tab with 1 hand and twisting off the top of the tobramycin inhalation solution ampule with the other hand (See Figure I). Be careful not to squeeze the tobramycin inhalation solution ampule until you are ready to empty all the medicine into the Nebulizer Cup.
(Figure I)
Step 12: Squeeze all the medicine of the tobramycin inhalation solution ampule into the Nebulizer Cup (See Figure J).
(Figure J)
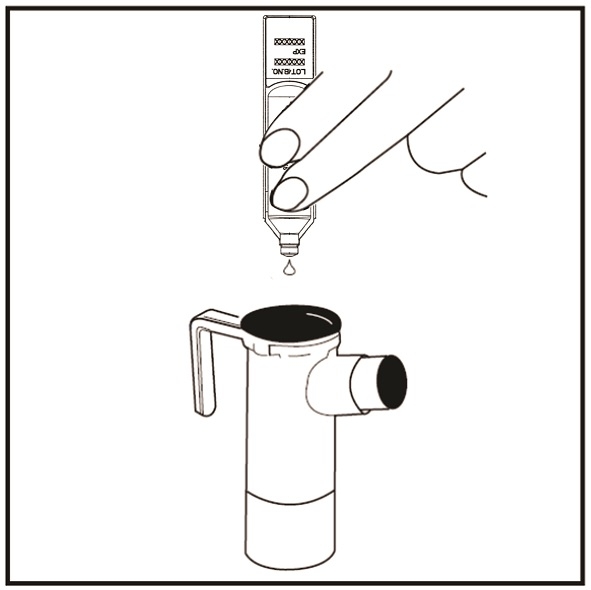
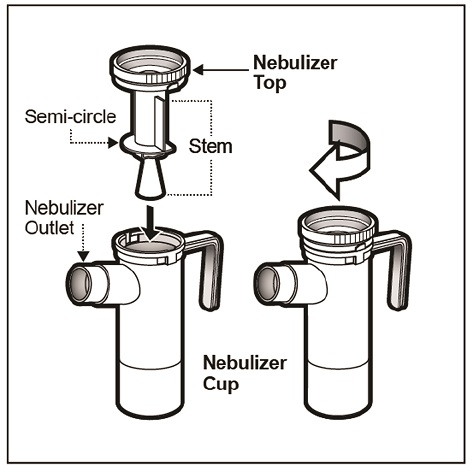
Step 13: Replace the Nebulizer Top. To replace the Nebulizer Top insert the Nebulizer Top into the Nebulizer Cup with the semi-circle halfway down the stem of the Nebulizer Top facing the Nebulizer Outlet. Turn the Nebulizer Top clockwise until securely fastened to the nebulizer Cup. (See Figure K).
(Figure K)
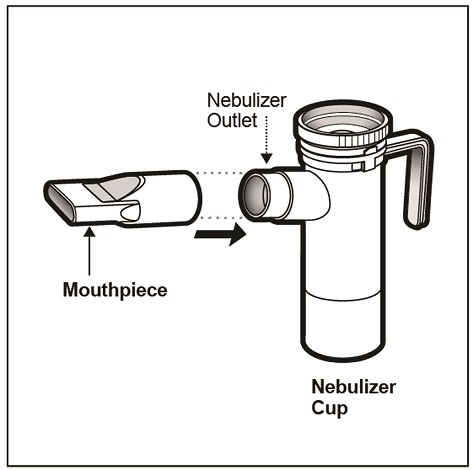
Step 14: Push the Mouthpiece straight onto the Nebulizer Outlet (See Figure L).
(Figure L)
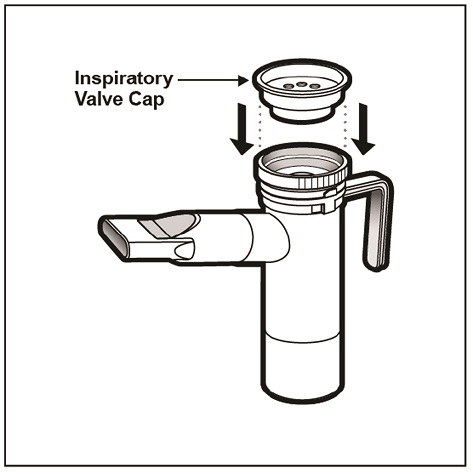
Step 15: Firmly push the Inspiratory Valve Cap straight down onto the Nebulizer Top (See Figure M). The Inspiratory Valve Cap will fit tightly.
(Figure M)
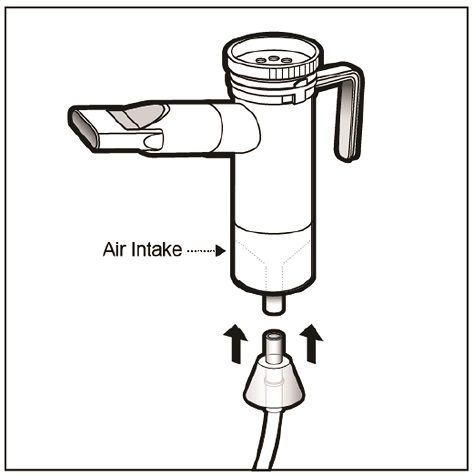
Step 16: Hold the Nebulizer Cup upright and firmly push the free end of the tubing from the compressor to the Air Intake on the bottom of the Nebulizer Cup (See Figure N). Make sure to keep the Nebulizer Cup upright.
(Figure N)
Giving your Tobramycin Inhalation Solution Inhalation Therapy
Step 17: Turn on the compressor (See Figure O).
(Figure O)
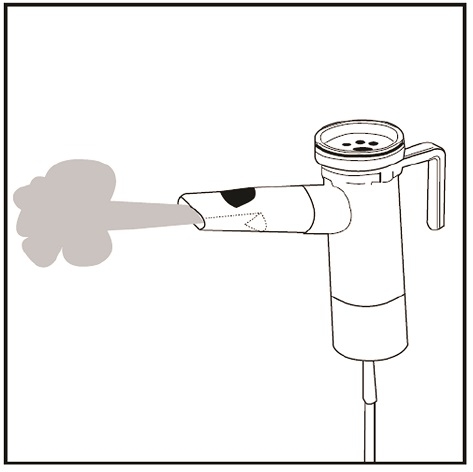
Step 18: Check for a steady mist from the Mouthpiece (See Figure P). If there is no mist, check all tubing connections and make sure that the compressor is working properly.
(Figure P)
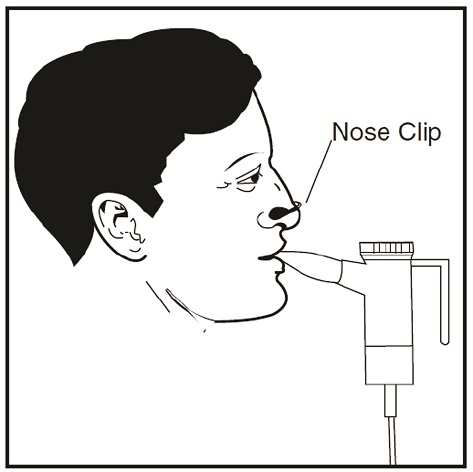
Step 19: Sit or stand in an upright position that will allow you to breathe normally. Place the Mouthpiece between your teeth and on top of your tongue and breathe normally only through your mouth (See Figure Q). Nose clips may help you breathe through your mouth and not through your nose. Do not block the airflow with your tongue.
(Figure Q)
Step 20: Keep breathing in your tobramycin inhalation solution medicine for at least 15 minutes to get your full dose. Continue therapy until all your tobramycin inhalation solution medicine is gone, and there is no longer any mist being made. You may hear a sputtering sound coming from the Mouthpiece when the Nebulizer Cup is empty. The entire tobramycin inhalation solution therapy should take about 15 minutes to complete.
If you are interrupted, need to cough or rest during your tobramycin inhalation solution treatment, turn off the compressor to save your medicine. Turn the compressor back on when you are ready to restart your treatment.
Follow the nebulizer cleaning and disinfecting instructions after completing your therapy.
After your Tobramycin Inhalation Solution Inhalation Therapy
Cleaning Your Nebulizer
To reduce the risk of infection, illness or injury from contamination, you must thoroughly clean all parts of the nebulizer as instructed after each treatment. Never use a nebulizer with a clogged nozzle. If the nozzle is clogged, no aerosol mist is made, and your therapy will not be as effective. Replace the nebulizer if clogging occurs.
1) Remove tubing from nebulizer and disassemble nebulizer parts.
2) Wash all parts (except tubing) with warm water and liquid dish soap.
3) Rinse thoroughly with warm water and shake out water.
4) Air dry or hand dry nebulizer parts on a clean, lint-free cloth. Reassemble nebulizer when dry, and store. You can also wash all parts of the nebulizer in a dishwasher (except tubing).
1) Place the nebulizer parts in a dishwasher basket.
2) Place the dishwasher basket on the top rack of the dishwasher.
3) Remove and dry the parts when the cycle is complete.
Disinfecting Your Nebulizer
Your nebulizer is for your use only. Do not share your nebulizer with other people. You must disinfect the nebulizer every other treatment day. Failure to disinfect the nebulizer every other treatment day could lead to serious or fatal illness.
Clean the nebulizer as described above. Every other treatment day, disinfect the nebulizer parts (except tubing) by boiling them in water for a full 10 minutes. Dry parts on a clean, lint-free cloth.
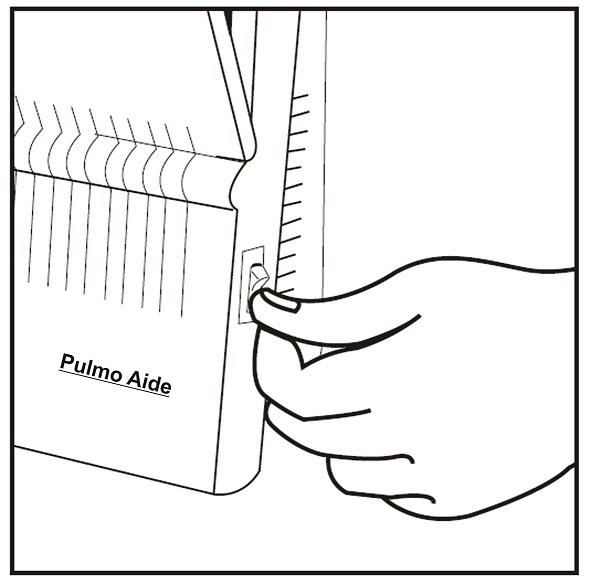
Care and Use of Your Pulmo-Aide Compressor
Follow the manufacturer instructions for care and use of your compressor.
Filter Change:
- DeVilbiss Compressor filters should be changed every 6 months or sooner if the filter turns completely gray in color.
- With power switch in the "Off" position, unplug power cord from wall outlet.
- Wipe outside of the compressor cabinet with a clean, damp cloth every few days to keep dust free.
Caution: Do not submerge in water because this will damage the compressor.
How should I store tobramycin inhalation solution?
- Store tobramycin inhalation solution ampules in a refrigerator between 36°F to 46°F (2°C to 8°C) until needed.
- You may store the tobramycin inhalation solution ampules in the foil pouches (opened or unopened) at room temperature 77°F (25°C) for up to 28 days.
- Do not use tobramycin inhalation solution ampules if they have been stored at room temperature for more than 28 days.
- Protect tobramycin inhalation solution ampules from light.
Keep tobramycin inhalation solution and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Additional Information
Nebulizer: 1-800-327-8632
Compressor: 1-800-338-1988
Tobramycin Inhalation Solution: 1-888-995-4337
Brand listed are trademark of their respective owners
Manufactured for:
Lifestar Pharma LLC
1200 MacArthur Blvd.
Mahwah, NJ 07430 USA
Product of Hungary
Revised: 03/2023, H-01
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL
NDC: 70756-604-44
Tobramycin Inhalation Solution, USP
300 mg/5 mL
For Inhalation Only by Nebulizer
Contains No Preservatives
Rx Only
4 Single-Dose 5 mL Ampules per Pouch
Store In Refrigerator
Sterile

NDC: 70756-604-56
Tobramycin Inhalation Solution, USP
300 mg/5 mL
For Inhalation Only by Nebulizer
56 (14 x 4) Single-Dose Ampules (28-Day Supply)
(4 Single-Dose 5 mL Ampules per Pouch,
14 Pouches per Carton)
Contains No Preservatives
Sterile
Store In Refrigerator
Rx only

-
INGREDIENTS AND APPEARANCE
TOBRAMYCIN
tobramycin solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70756-604 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOBRAMYCIN (UNII: VZ8RRZ51VK) (TOBRAMYCIN - UNII:VZ8RRZ51VK) TOBRAMYCIN 300 mg in 5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) SULFURIC ACID (UNII: O40UQP6WCF) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70756-604-56 14 in 1 CARTON 06/30/2023 1 NDC: 70756-604-44 4 in 1 POUCH 1 5 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA214478 06/30/2023 Labeler - Lifestar Pharma LLC (080268943) Registrant - Mankind Pharma Limited (915834068) Establishment Name Address ID/FEI Business Operations Mankind Pharma Limited 916512493 MANUFACTURE(70756-604) , ANALYSIS(70756-604) , PACK(70756-604)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.