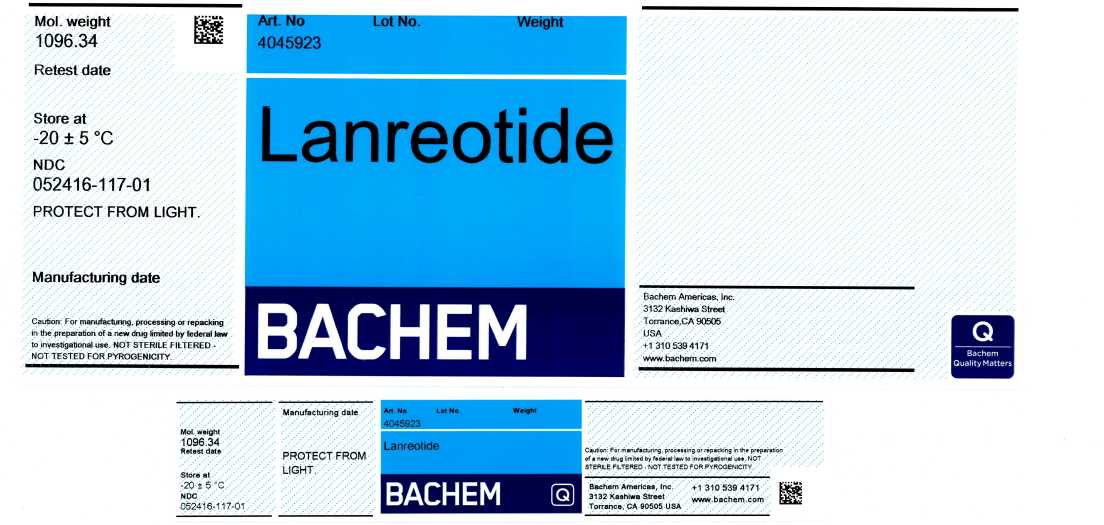
Product Listing for Lanreotide
Lanreotide by
Drug Labeling and Warnings
Lanreotide by is a Other medication manufactured, distributed, or labeled by Bachem Americas, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LANREOTIDE- lanreotide powder
Bachem Americas, Inc.
----------
Product Listing for Lanreotide
| LANREOTIDE
lanreotide powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bachem Americas, Inc. (077243640) |
Revised: 6/2019
Document Id: 8b88c69a-9b86-908d-e053-2995a90ae231
Set id: 557cad64-e8b5-47ca-9d2b-fedd642d1afd
Version: 2
Effective Time: 20190618
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.