KYNMOBI- apomorphine hydrochloride film, soluble KYNMOBI- apomorphine hydrochloride kit
KYNMOBI by
Drug Labeling and Warnings
KYNMOBI by is a Prescription medication manufactured, distributed, or labeled by Sunovion Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use KYNMOBI safely and effectively. See full prescribing information for KYNMOBI.
KYNMOBI™ (apomorphine hydrochloride) sublingual film
Initial U.S. Approval: 2004INDICATIONS AND USAGE
KYNMOBI is a non-ergoline dopamine agonist indicated for the acute, intermittent treatment of “off” episodes in patients with Parkinson's disease (1)
DOSAGE AND ADMINISTRATION
- For sublingual administration only (2.1)
- Dose initiation should be supervised by a healthcare provider (2.1, 2.3)
- Treatment with a concomitant antiemetic, e.g. trimethobenzamide, is recommended, beginning 3 days prior to initial dose of KYNMOBI (2.1, 5.1)
- The dose range for KYNMOBI is 10 mg to 30 mg per dose, administered sublingually, as needed (2.2)
- KYNMOBI doses should be separated by at least 2 hours (2.2)
- Maximum of 5 doses per day; maximum single dose is 30 mg (2.2, 2.3)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Nausea and vomiting may occur (2.1, 5.1)
- Falling asleep during activities of daily living and daytime somnolence may occur, discontinue KYNMOBI if occurs (5.2)
- Syncope and hypotension/orthostatic hypotension may occur, monitor blood pressure (5.3)
- Oral mucosal irritation may occur, which may require pausing or discontinuing treatment (5.4)
- Falls may occur, or increase (5.6)
- May cause hallucinations and psychotic-like behavior (5.7)
- May cause impulse control and impulsive behaviors; consider dose reduction or discontinuing KYNMOBI if occurs (5.8)
- Withdrawal-emergent hyperpyrexia and confusion may occur with rapid dose reduction or withdrawal (5.9)
- May prolong QTc and cause torsades de pointes or sudden death; consider risk factors prior to initiation (5.10)
ADVERSE REACTIONS
Most common adverse reactions (incidence at least 10% in patients treated with KYNMOBI and with an incidence greater than placebo) were nausea, oral/pharyngeal soft tissue swelling, oral/pharyngeal soft tissue pain and paraesthesia, dizziness, and somnolence (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sunovion Pharmaceuticals Inc. at 1-877-737-7226 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosing Information
2.3 Dose Titration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Nausea and Vomiting
5.2 Falling Asleep During Activities of Daily Living and Somnolence
5.3 Hypersensitivity
5.4 Syncope / Hypotension / Orthostatic Hypotension
5.5 Oral Mucosal Irritation
5.6 Falls
5.7 Hallucinations / Psychotic-Like Behavior
5.8 Impulse Control/Compulsive Behaviors
5.9 Withdrawal-Emergent Hyperpyrexia and Confusion
5.10 QTc Prolongation and Potential for Proarrhythmic Effects
5.11 Fibrotic Complications
5.12 Priapism
5.13 Retinal Pathology in Albino Rats
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 5HT3 Antagonists
7.2 Antihypertensive Medications and Vasodilators
7.3 Alcohol
7.4 Dopamine Antagonists
7.5 Drugs Prolonging the QT/QTc Interval
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Dose initiation should be supervised by a healthcare provider [see Dosage and Administration (2.3)].
KYNMOBI must be administered whole. Do not to cut, chew, or swallow KYNMOBI. KYNMOBI will disintegrate in about 3 minutes.
Because of the high incidence of nausea and vomiting with KYNMOBI when administered at recommended doses, an antiemetic (e.g., trimethobenzamide 300 mg three times a day), beginning 3 days prior to the initial dose of KYNMOBI, is recommended. Treatment with the antiemetic should only be continued as long as necessary to control nausea and vomiting, and generally no longer than two months after initiation of treatment with KYNMOBI [see Contraindications (4) and Warnings and Precautions (5.1)].
Based on reports of profound hypotension and loss of consciousness when apomorphine was administered with ondansetron, the concomitant use of apomorphine with drugs of the 5HT3 antagonist class including antiemetics (for example, ondansetron, granisetron, dolasetron, palonosetron) and alosetron are contraindicated [see Contraindications (4)].
2.2 Dosing Information
The dose range for KYNMOBI is 10 mg to 30 mg per dose, administered sublingually, as needed, for the acute, intermittent treatment of “off” episodes.
Doses should be separated by at least 2 hours. If a single dose of KYNMOBI is ineffective for a particular “off” episode, a second dose should not be given for that “off” episode. The efficacy or safety of administering a second dose for a single “off” episode has not been studied.
Do not administer more than 5 doses per day.
The maximum single dose of KYNMOBI is 30 mg.
2.3 Dose Titration
The initial dose is 10 mg. Dose initiation should occur when the patient is in an “off” state and in a setting where a healthcare provider can monitor blood pressure and pulse. In clinical studies of KYNMOBI, the “off” state was achieved by instructing patients to not take their regular morning dose of carbidopa/levodopa or any other adjunctive Parkinson's disease medications, and to take their last dose of carbidopa/levodopa and any other adjunctive Parkinson's disease medications no later than midnight the night before [see Clinical Studies (14)].
If the patient tolerates the 10 mg dose, and responds adequately, the starting dose should be 10 mg, used on an as needed basis, up to 5 times per day, to treat “off” episodes. If the dose is tolerated but the response is insufficient, the patient's usual Parkinson's disease medications should be resumed and up-titration with KYNMOBI continued generally within 3 days. Increase dosage by increments of 5 mg and assess response. Continue to titrate in a similar manner, under the supervision of a healthcare provider, until an effective and tolerable dose is achieved [see Dosage and Administration (2.2) and Clinical Studies (14)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
KYNMOBI is contraindicated in patients:
- Using concomitant 5HT3 antagonists, including antiemetics (e.g., ondansetron, granisetron, dolasetron, palonosetron) and alosetron [see Drug Interactions (7.1)]. There have been reports of profound hypotension and loss of consciousness when subcutaneous apomorphine was administered with a 5HT3 antagonist.
- With hypersensitivity/allergic reaction to apomorphine or to any of the ingredients of KYNMOBI. Angioedema or anaphylaxis may occur [see Warnings and Precautions (5.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Nausea and Vomiting
KYNMOBI may cause nausea and vomiting when administered at recommended doses. Because of the high incidence of nausea and vomiting with KYNMOBI when administered at recommended doses, an antiemetic, e.g., trimethobenzamide 300 mg three times a day, is recommended beginning 3 days prior to the initial dose of KYNMOBI. Treatment with the antiemetic should only be continued as long as necessary to control nausea and vomiting, and generally no longer than two months after initiation of treatment with KYNMOBI [see Dosage and Administration (2.1)].
In Study 1 [see Clinical Studies (14)], treatment with an antiemetic (i.e., trimethobenzamide hydrochloride; 300 mg by mouth three times daily) was required beginning 3 days before starting KYNMOBI; however, it could be discontinued during the maintenance phase. During the titration phase of Study 1, nausea was reported as an adverse reaction by 21% of patients treated with KYNMOBI, while vomiting was reported as an adverse reaction by 4% of patients treated with KYNMOBI. During the maintenance phase of Study 1, nausea was reported as an adverse reaction by 28% of patients treated with KYNMOBI, compared with 4 % of patients who received placebo. During the maintenance phase of Study 1, vomiting was reported as an adverse reaction by 7% of patients treated with KYNMOBI, compared with 0 % of patients who received placebo. Nausea or vomiting was the reason for withdrawal from the study in 2% of patients treated with KYNMOBI during the titration phase and 2% of patients treated with KYNMOBI during the maintenance phase.
Concomitantly administered antiemetic drugs other than trimethobenzamide have not been studied. 5HT3 antagonist antiemetics are contraindicated [see Contraindications (4)]. Antiemetics with anti-dopaminergic actions (e.g., haloperidol, chlorpromazine, promethazine, prochlorperazine, metoclopramide) have the potential to worsen symptoms in patients with Parkinson's disease and should be avoided [see Drug Interactions (7.4)].
5.2 Falling Asleep During Activities of Daily Living and Somnolence
Patients treated with dopaminergic medications, including apomorphine, have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles, which sometimes has resulted in accidents. Patients may not perceive warning signs, such as excessive drowsiness, or they may report feeling alert immediately prior to the event.
During the titration phase of Study 1, somnolence was reported as an adverse reaction in 11% of patients treated with KYNMOBI. During the maintenance phase of Study 1, somnolence was reported as an adverse reaction in 13% of patients treated with KYNMOBI, compared with 2% of patients who received placebo.
Prescribers should reassess patients for drowsiness or sleepiness, especially since some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities.
Before initiating treatment with KYNMOBI, advise patients of the risk of drowsiness and ask them about factors that could increase the risk with KYNMOBI, such as concomitant sedating medications and the presence of sleep disorders. If a patient develops significant daytime sleepiness or falls asleep during activities that require active participation (e.g., conversations, eating, etc.), KYNMOBI should ordinarily be discontinued. If a decision is made to continue KYNMOBI, patients should be advised not to drive and to avoid other potentially dangerous activities. There is insufficient information to determine whether dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
5.3 Hypersensitivity
Oral soft tissue swelling (lips, tongue, gingiva, and mouth) was reported as adverse reaction in 15% of patients treated with KYNMOBI during the maintenance phase of Study 1, compared with 0% of patients who received placebo; 11% of patients discontinued KYNMOBI because of this event.
Swelling of the face, oral allergy syndrome, hypersensitivity, or urticaria were reported as an adverse reaction in 6% of patients treated with KYNMOBI during the maintenance phase of Study 1, compared with 0% of patients who received placebo; 4% of patients discontinued KYNMOBI because of this event.
It is not known whether these events are related to apomorphine, sodium metabisulfite, or another KYNMOBI excipient.
KYNMOBI rechallenge is not generally recommended after discontinuation as oral adverse reactions may recur and may be more severe than the initial reaction.
Sulfite Sensitivity
KYNMOBI contains sodium metabisulfite, a sulfite that may cause allergic-type reactions, including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people.
5.4 Syncope / Hypotension / Orthostatic Hypotension
KYNMOBI may cause syncope, hypotension, or orthostatic hypotension. During the titration phase of Study 1, syncope, pre-syncope, hypotension, or orthostatic hypotension were reported as adverse reactions in 4% of patients. During the maintenance phase of Study 1, syncope, pre-syncope, hypotension, or orthostatic hypotension were reported as adverse reactions in 2% of patients treated with KYNMOBI, compared with 0% of patients who received placebo.
During the maintenance phase of Study 1, systolic orthostatic hypotension (reduction of 20 mmHg or more in standing minus supine/sitting systolic blood pressure) or diastolic hypotension (10 mmHg or more for standing minus supine/sitting diastolic blood pressure) occurred in 43% of patients treated with KYNMOBI, and in 36% of patients who received placebo.
Patients treated with KYNMOBI should receive an assessment for hypotension / orthostatic hypotension, especially if they have a history of hypotension or cardiovascular disease, or if they are currently using antihypertensive medication. Inform patients of the risk of orthostatic hypotension.
The hypotensive effects of KYNMOBI may be increased by the concomitant use of alcohol, antihypertensive medications, and vasodilators (especially nitrates). Patients should avoid alcohol when using KYNMOBI [see Drug Interactions (7.3), Clinical Pharmacology (12.3)]. Patients taking KYNMOBI should lie down before and after taking sublingual nitroglycerin [see Drug Interactions (7.2), Clinical Pharmacology (12.3)].
Monitor patients taking concomitant antihypertensive medications for hypotension and orthostatic hypotension [see Drug Interactions (7.2, 7.3)].
5.5 Oral Mucosal Irritation
During the titration phase of Study 1, oral mucosal ulceration or stomatitis were reported as adverse reactions in 2% of patients treated with KYNMOBI. During the maintenance phase of Study 1, oral mucosal ulceration or stomatitis were reported as adverse reactions in 7% of patients treated with KYNMOBI, compared with 0% of patients who received placebo [see Adverse Reactions (6.1)].
During the titration of Study 1, oral soft tissue pain or paresthesia were reported as adverse reactions in 2% of patients treated with KYNMOBI. During the maintenance phase of Study 1, oral soft tissue pain or paresthesia were reported as adverse reactions in 13% of patients treated with KYNMOBI, compared with 2% of patients who received placebo.
In general, oral mucosal irritation reactions were mild to moderate severity, and usually resolved with treatment discontinuation.
KYNMOBI rechallenge is not generally recommended after discontinuation as oral adverse reactions may recur and be more severe than the initial reaction.
Hypersensitivity adverse reactions may also occur during treatment with KYNMOBI [see Warnings and Precautions (5.3)].
5.6 Falls
Patients with Parkinson's disease are at risk of falling because of underlying postural instability, possible autonomic instability, and syncope caused by the blood pressure lowering effects of the drugs used to treat Parkinson's disease [see Clinical Pharmacology (12.2)]. KYNMOBI might increase the risk of falling by simultaneously lowering blood pressure and altering mobility [see Warnings and Precautions (5.4)].
During the titration period of Study 1, falls were reported as adverse reaction in 4% of patients treated with KYNMOBI. During the maintenance period of Study 1, falls were reported as adverse reaction in 6% of patients treated with KYNMOBI, compared with 2% of patients who received placebo.
5.7 Hallucinations / Psychotic-Like Behavior
During the maintenance phase of Study 1, hallucinations, delusions, disorientation, or confusion were reported as adverse reactions in 6% of patients treated with KYNMOBI, compared with 2% of patients who received placebo. No patient developed hallucinations or psychotic-like behavior during the titration phase.
A total of 4% of patients treated with KYNMOBI discontinued treatment because of disorientation, confusional state, or delusions, compared with 2% of patients who received placebo.
Postmarketing reports with subcutaneous apomorphine indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic-like behavior after starting or increasing the dose of apomorphine. Other drugs prescribed to improve the symptoms of Parkinson's disease can have similar effects on thinking and behavior. This abnormal thinking and behavior can consist of one or more of a variety of manifestations, including paranoid ideation, delusions, hallucinations, confusion, disorientation, aggressive behavior, agitation, and delirium.
Patients with a major psychotic disorder should ordinarily not be treated with apomorphine because of the risk of exacerbating psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson's disease and may decrease the effectiveness of KYNMOBI [see Drug Interactions (7.4)].
5.8 Impulse Control/Compulsive Behaviors
Case reports suggest that patients can experience intense urges to gamble, increased sexual urges, intense urges to spend money uncontrollably, and other intense urges and the inability to control these urges while taking one or more medications, including KYNMOBI, that increase central dopaminergic tone. In some cases, although not all, these urges were reported to have stopped when the dose was reduced, or the medication was discontinued. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending, binge eating or other urges while being treated with KYNMOBI. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking KYNMOBI.
5.9 Withdrawal-Emergent Hyperpyrexia and Confusion
A symptom complex resembling the neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, elevated serum creatine kinase, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in antiparkinsonian therapy.
5.10 QTc Prolongation and Potential for Proarrhythmic Effects
At exposures achieved with therapeutic doses of subcutaneous apomorphine, a dose-related prolongation of QTc has been observed [see Clinical Pharmacology (12.2)]. Although the extent of the exposure and the Cmax of apomorphine are lower following the maximum recommended dose of KYNMOBI (30 mg) than following the maximum recommended dose of subcutaneous apomorphine (6 mg), QTc prolongation with KYNMOBI cannot be excluded.
Drugs that prolong the QTc interval have been associated with torsades de pointes and sudden death. The relationship of QTc prolongation to torsades de pointes is clearest for larger increases (20 msec and greater), but it is possible that smaller QTc prolongations may also increase risk, or increase it in susceptible individuals, such as those with hypokalemia, hypomagnesemia, bradycardia, concomitant use of other drugs that prolong the QTc interval, or genetic predisposition (e.g., congenital prolongation of the QT interval). Although torsades de pointes has not been observed in association with the use of KYNMOBI at recommended doses in clinical studies, experience is too limited to rule out an increased risk. Palpitations and syncope may signal the occurrence of an episode of torsades de pointes.
The risks and benefits of KYNMOBI treatment should be considered prior to initiating treatment with KYNMOBI in patients with risk factors for prolonged QTc.
5.11 Fibrotic Complications
Cases of retroperitoneal fibrosis, pulmonary infiltrates, pleural effusion, pleural thickening, and cardiac valvulopathy have been reported in some patients treated with ergot-derived dopaminergic agents. While these complications may resolve when the drug is discontinued, complete resolution does not always occur. Although these adverse reactions are believed to be related to the ergoline structure of these dopamine agonists, whether other, nonergot derived dopamine agonists, such as KYNMOBI, can cause these reactions is unknown.
5.12 Priapism
Apomorphine may cause prolonged painful erections in some patients. Severe priapism may require surgical intervention.
5.13 Retinal Pathology in Albino Rats
In a 2-year carcinogenicity study of apomorphine in albino rat, retinal atrophy was detected at all subcutaneous doses tested (up to 0.8 mg/kg/day or 2 mg/kg/day in males or females, respectively). Retinal atrophy/degeneration has been observed in albino rats treated with other dopamine agonists for prolonged periods (generally during 2-year carcinogenicity studies). Retinal findings were not observed in a 39-week subcutaneous toxicity study of apomorphine in monkey at doses up to 1.5 mg/kg/day. The clinical significance of the finding in rat has not been established but cannot be disregarded because disruption of a mechanism that is universally present in vertebrates (e.g., disk shedding) may be involved.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in more detail in the Warnings and Precautions section of labeling:
- Nausea and Vomiting [see Warnings and Precautions (5.1)]
- Falling Asleep During Activities of Daily Living and Somnolence [see Warnings and Precautions (5.2)]
- Hypersensitivity [see Warnings and Precautions (5.3)]
- Syncope/Hypotension/Orthostatic Hypotension [see Warnings and Precautions (5.4)]
- Oral Mucosal Irritation [see Warnings and Precautions (5.5)]
- Falls [see Warnings and Precautions (5.6)]
- Hallucinations/Psychotic Behavior [see Warnings and Precautions (5.7)]
- Impulse Control/Compulsive Behaviors [see Warnings and Precautions (5.8)]
- Withdrawal-Emergent Hyperpyrexia and Confusion [see Warnings and Precautions (5.9)]
- QTc Prolongation and Potential for Proarrhythmic Effects [see Warnings and Precautions (5.10)]
- Fibrotic Complications [see Warnings and Precautions (5.11)]
- Priapism [see Warnings and Precautions (5.12)]
- Oral Adverse Events [see Warnings and Precautions (5.13)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
KYNMOBI safety data presented below is derived from a randomized, double blind, placebo-controlled study in patients with Parkinson's disease (Study 1) [see Clinical Studies (14)]. Study 1 included a titration phase, in which 141 patients received at least one dose of KYNMOBI, followed by a placebo-controlled 12-week maintenance phase. The mean age of patients in Study 1 was 63 years (range 43 to 86 years); 63% of patients were male, and 93% were Caucasian.
The most common adverse reactions (incidence at least 10% in patients treated with KYNMOBI and with an incidence greater than placebo) were nausea, oral/pharyngeal soft tissue swelling, oral/pharyngeal soft tissue pain and paraesthesia, dizziness, and somnolence.
Adverse reactions led to discontinuation of KYNMOBI in 9% of patients in the titration phase, and 28% of patients in the maintenance phase, compared with 7% of patients on placebo (in the maintenance phase). The most common adverse reactions leading to discontinuation during the maintenance phase were oral/pharyngeal soft tissue swelling, oral mucosal erythema, and nausea/vomiting.
Table 1 presents the adverse reactions that occurred in at least 5% of patients treated with KYNMOBI during the maintenance phase of Study 1, and with an incidence greater than in patients who received placebo.
Table 1: Adverse Reactions Reported by at Least 5% of Patients Treated with KYNMOBI during the Maintenance Phase of Study 1, and with an Incidence Greater than Placebo Titration Maintenance KYNMOBI
(N=141)
%KYNMOBI
(N=54)
%Placebo
(N=55)
%1 Includes lip swelling, lip edema, oropharyngeal swelling, gingival edema, edema mouth, swollen tongue, and
pharyngeal edema
2 Includes throat irritation, glossodynia, oral pain, oral paresthesia, oropharyngeal pain, gingival pain, and oral
hypoesthesia
3 Includes lip ulceration, oral mucosal blistering, stomatitis, cheilitis, and tongue ulceration
4 Includes hypersensitivity, swelling face, oral allergy syndrome and urticaria
Gastrointestinal disorders
Nausea
Oral/pharyngeal soft tissue swelling1
Oral/pharyngeal soft tissue pain and paraesthesia2
Oral ulceration and stomatitis3
Oral mucosal erythema
Vomiting
Dry mouth
21
1
2
2
4
4
1
28
15
13
7
7
7
6
4
0
2
0
4
0
0Nervous system disorders
Somnolence
Dizziness
Headache
11
11
8
13
9
6
2
0
0Respiratory, thoracic, and mediastinal disorders Rhinorrhea 6 7 0 General disorders and administration site conditions
Fatigue 3 7 0 Injury, poisoning, and procedural complications Fall
Laceration4
16
62
0Skin and subcutaneous tissue disorders
Hyperhidrosis
4
6
4Immune system disorders
Hypersensitivity4
0
6
0Less Common Adverse Reactions
Other adverse reactions including hallucinations, delusions, and impulse control disorder have been reported in patients treated with KYNMOBI [see Warnings and Precautions (5.7, 5.8)].
Vital Sign Changes
Blood Pressure
Decreases in blood pressure have been observed in patients treated with KYNMOBI. During the titration phase of Study 1, syncope, pre-syncope, hypotension, or orthostatic hypotension were reported as adverse reaction in 4% of patients treated with KYNMOBI. During the maintenance phase of Study 1, syncope, pre-syncope, hypotension, or orthostatic hypotension were reported as adverse reaction in 2 % of patients treated with KYNMOBI, compared with 0% of patients who received placebo [see Warnings and Precautions (5.4) and Clinical Pharmacology (12.2)].
-
7 DRUG INTERACTIONS
7.1 5HT3 Antagonists
Based on reports of profound hypotension and loss of consciousness when subcutaneous apomorphine was administered with ondansetron, the concomitant use of KYNMOBI with 5HT3 antagonists, including antiemetics (e.g., ondansetron, granisetron, palonosetron) and alosetron, is contraindicated [see Warnings and Precautions (5.4)].
7.2 Antihypertensive Medications and Vasodilators
In a study of healthy subjects, concomitant administration of 0.4 mg sublingual nitroglycerin with subcutaneous apomorphine caused greater decreases in blood pressure than with subcutaneous apomorphine alone [see Clinical Pharmacology (12.3)].
Patients taking KYNMOBI should lie down before and after taking sublingual nitroglycerin [see Warnings and Precautions (5.4)].
7.3 Alcohol
In a study of healthy subjects, concomitant administration of high dose (0.6 g/kg) or low dose (0.3 g/kg) ethanol with subcutaneous apomorphine caused greater decreases in blood pressure than with subcutaneous apomorphine alone [see Clinical Pharmacology (12.3)].
Patients should avoid drinking alcohol after using KYNMOBI [see Warnings and Precautions (5.4)].
7.4 Dopamine Antagonists
Since KYNMOBI is a dopamine agonist, it is possible that concomitant use of dopamine antagonists, such as the neuroleptics (e.g., phenothiazines, butyrophenones, thioxanthenes) or metoclopramide, may diminish the effectiveness of KYNMOBI. Antiemetics with anti-dopaminergic actions should be avoided [see Warnings and Precautions (5.1)]. Patients with major psychotic disorders receiving neuroleptics should be treated with dopamine agonists only if the potential benefits outweigh the risks [see Warnings and Precautions (5.7)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with use of KYNMOBI in pregnant women. In animal reproduction studies, apomorphine had adverse developmental effects in rats (increased neonatal deaths) and rabbits (increased incidence of malformation) when administered during pregnancy at clinically relevant doses. These doses were also associated with maternal toxicity [see Data]. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
No adverse developmental effects were observed when apomorphine (0.3, 1, or 3 mg/kg/day) was administered by subcutaneous injection to pregnant rats throughout organogenesis. Administration of apomorphine (0.3, 1, or 3 mg/kg/day) by subcutaneous injection to pregnant rabbits throughout organogenesis resulted in an increased incidence of malformations of the heart and/or great vessels at the mid and high doses; maternal toxicity was observed at the highest dose tested.
Apomorphine (0.3, 1, or 3 mg/kg/day), administered by subcutaneous injection to females throughout gestation and lactation, resulted in increased offspring mortality at the highest dose tested, which was associated with maternal toxicity. There were no effects on developmental parameters or reproductive performance in surviving offspring.
8.2 Lactation
Risk Summary
There are no data on the presence of apomorphine in human milk, the effects of apomorphine on the breastfed infant, or the effects of apomorphine on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for KYNMOBI and any potential adverse effects on the breastfed infant from KYNMOBI or from the underlying maternal condition.
8.5 Geriatric Use
Clinical studies of KYNMOBI did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In Study 1, 78 patients below 65 years of age and 63 patients 65 years of age or older received at least one dose of KYNMOBI. Clinical experience with subcutaneous use of apomorphine has shown that the following adverse reactions were reported more frequently in patients 65 years of age or older compared to patients less than 65 years of age: confusion; hallucinations; serious adverse reactions (life-threatening events or events resulting in hospitalization and/or increased disability); fall (experiencing bone and joint injuries); cardiovascular events; respiratory disorders; gastrointestinal events; and discontinuation of treatment as a result of one or more adverse reactions.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Renal Impairment
Avoid use of KYNMOBI in patients with severe and end-stage renal disease (ESRD) (CLcr <30 mL/min). No dosage adjustment is required for patients with mild or moderate renal impairment. However, because of a potential for increased exposure, titrate KYNMOBI under medical supervision [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Avoid use of KYNMOBI in patients with severe hepatic impairment (Child-Pugh Class C). No dosage adjustment is required for patients with mild or moderate hepatic impairment (Child-Pugh Class A and B). However, because of a potential for increased exposure, titrate KYNMOBI under medical supervision [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
In premarketing clinical experience, KYNMOBI did not reveal any tendency for a withdrawal syndrome or any drug-seeking behavior. However, there are rare postmarketing reports of abuse of medications containing apomorphine. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. In general, these reports for apomorphine consist of patients taking increasing doses of medication in order to achieve a euphoric state.
-
11 DESCRIPTION
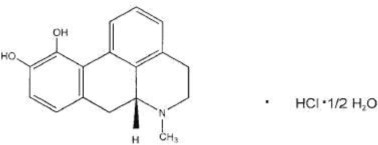
KYNMOBI (apomorphine hydrochloride) sublingual film contains apomorphine hydrochloride, a non-ergoline dopamine agonist. Apomorphine hydrochloride is chemically designated as 6aβ-Aporphine-10,11-diol hydrochloride hemihydrate with a molecular formula of C17H17NO2∙HCl∙½ H2O. Its structural formula and molecular weight are:
Figure 1: Structural Formula and Molecular Weight of Apomorphine Hydrochloride
The molecular weight is 312.79 (hydrochloride hemihydrate salt).
Apomorphine hydrochloride is white to grayish glistening crystals or white powder that is sparingly soluble in water and alcohol at ambient temperature.
KYNMOBI is intended for sublingual administration only and is available in five dosage strengths. Each film contains 10 mg, 15 mg, 20 mg, 25 mg, or 30 mg of apomorphine hydrochloride (equivalent to 8.8 mg, 13.2 mg, 17.6 mg, 22.0 mg, and 26.4 mg of apomorphine, respectively). Each film also contains the following inactive ingredients: disodium EDTA, dihydrate, FD&C Blue #1, glycerol, glyceryl monostearate, hydroxyethyl cellulose, hypromellose, maltodextrin, (-)-menthol, pyridoxine hydrochloride, sodium hydroxide, sodium metabisulfite, sucralose, and white ink.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
KYNMOBI is a non-ergoline dopamine agonist with high in vitro binding affinity for the dopamine D4 receptor, and moderate affinity for the dopamine D2, D3, and D5, and adrenergic α1D, α2B, α2C receptors. The precise mechanism of action of KYNMOBI as a treatment for “off” episodes associated with Parkinson's disease is unknown, although it is believed to be due to stimulation of post-synaptic dopamine D2-type receptors within the caudate-putamen in the brain.
12.2 Pharmacodynamics
Cardiac Electrophysiology
In a thorough QT study with subcutaneous apomorphine at exposures similar to those achieved with the recommended subcutaneous apomorphine dosing (i.e, 6 mg), apomorphine resulted in a prolongation of QTcF of 10 msec (90% upper confidence interval of 16 msec). The thorough QT study also identified a significant exposure-response relationship between apomorphine concentration and QTcF.
Although the extent of the exposure and the Cmax of apomorphine are lower following the maximum recommended dose of KYNMOBI (30 mg) than following the maximum recommend dose of subcutaneous apomorphine (6 mg), QTc prolongation with KYNMOBI cannot be excluded.
Decreases in Blood Pressure
In Study 1, systolic orthostatic hypotension (reduction of 20 mmHg or more in standing minus supine/sitting systolic blood pressure) or diastolic hypotension (10 mmHg or more for standing minus supine/sitting diastolic blood pressure) occurred in 43% of patients treated with KYNMOBI, compared to 36% of patients who received placebo [see Warnings and Precautions (5.4) and Drug Interactions (7.2, 7.3)].
12.3 Pharmacokinetics
Absorption
Following sublingual administration of 15 mg of apomorphine, the time to maximum concentration (Tmax) ranged from 0.5 to 1 hour. Apomorphine exhibits less than dose proportional increase in exposures over a dose range of 10 mg to 35 mg (1.2 times the highest recommended dosage) following a single sublingual administration of KYNMOBI in patients with Parkinson's disease.
Distribution
Following sublingual administration of 15 mg of apomorphine, the geometric mean (CV%) of the apparent volume of distribution was 3630 L (66%).
Elimination
Metabolism
The major metabolic pathways for sublingual apomorphine are sulfation by multiple sulfotransferase (SULT) enzymes; glucuronidation by multiple glycosyltransferase (UGT) enzymes; N-demethylation catalyzed by multiple enzymes, including CYP2B6, CYP2C8, and CYP3A4/5; followed by conjugation. Metabolism of sublingual apomorphine results in three major inactive metabolites: apomorphine sulfate, apomorphine glucuronide, and norapomorphine glucuronide.
Specific Populations
The apparent clearance of apomorphine does not appear to be influenced by age, gender, race, weight, duration of Parkinson's disease, levodopa dose, use of antiemetic, or duration of therapy.
Renal Impairment
The clinical studies of KYNMOBI included patients with mild renal impairment (CLcr of ≥ 60 mL/min and <90 mL/min). There were no differences in apomorphine exposure after administration of KYNMOBI in patients with mild renal impairment as compared to patients with normal renal function (CLcr of ≥ 90 mL/min). Studies with KYNMOBI in patients with moderate to severe renal impairment have not been conducted.
In a study with subcutaneous apomorphine comparing patients with moderate renal impairment (as determined by estimated creatinine clearance) to healthy matched volunteers, the AUC0-∞ and Cmax values were increased by approximately 16% and 50%, respectively, following a single administration. The mean time to peak concentrations and the mean terminal half-life of apomorphine were unaffected by the renal status of the individual.
Since the Cmax and AUC0-∞ of apomorphine following the sublingual administration are lower as compared to the subcutaneous route of administration and the KYNMOBI dose is titrated individually, these changes are not expected to be clinically significant for patients with mild or moderate renal impairment [see Use in Specific Populations (8.6)].
Hepatic Impairment
Studies with KYNMOBI in patients with hepatic impairment have not been conducted.
In a study with subcutaneous apomorphine comparing patients with moderate hepatic impairment (as determined by the Child-Pugh classification method) to healthy matched volunteers, the AUC0-∞ and Cmax values were increased by approximately 10% and 25%, respectively, following a single administration. These changes are not expected to be clinically significant for patients with mild or moderate hepatic impairment [see Use in Specific Populations (8.7)].
Drug Interaction Studies
Carbidopa/levodopa
Levodopa pharmacokinetics were unchanged when subcutaneous apomorphine and levodopa were co-administrated in patients. However, motor response differences were significant. The threshold levodopa concentration necessary for an improved motor response was reduced significantly, leading to an increased duration of effect without a change in the maximal response to levodopa therapy.
Nitroglycerin
Co-administration of nitroglycerin (0.4 mg) with subcutaneous apomorphine in healthy subjects did not have a significant impact on the pharmacokinetics of apomorphine. However, concomitant administration of nitroglycerin (0.4 mg) with subcutaneous apomorphine caused greater decreases in blood pressure than with subcutaneous apomorphine alone [see Warnings and Precautions (5.4) and Drug Interactions (7.2)].
When nitroglycerin and subcutaneous apomorphine were concomitantly administered to healthy subjects, the mean largest decrease (the mean of each subject's largest drop in blood pressure measured within the 6-hour period following administration of subcutaneous apomorphine) in supine systolic and diastolic blood pressure (measured over 6 hours) was 9.7 mm Hg and 9.3 mm Hg, respectively. The mean largest decrease in standing systolic and diastolic blood pressure was 14.3 mm Hg and 13.5 mm Hg, respectively. Some individuals experienced very large decreases in standing systolic and diastolic blood pressure, up to a maximum decrease of 65 mm Hg and 43 mm Hg, respectively. In comparison, the mean largest decrease in supine systolic and diastolic blood pressure when subcutaneous apomorphine was administered alone was 6.1 mm Hg and 7.3 mm Hg, respectively, and in standing systolic and diastolic blood pressure was 6.7 mm Hg and 8.4 mm Hg, respectively.
A similar study has not been performed with KYNMOBI.
Ethanol
Co-administration of low dose ethanol (0.3 g/kg) with subcutaneous apomorphine in healthy subjects did not have a significant impact on the pharmacokinetics of apomorphine, but high dose ethanol (0.6 g/kg), equivalent to approximately 3 standardized alcohol-containing beverages, increased the Cmax of apomorphine by about 63%.
When high dose ethanol (0.6 g/kg) and subcutaneous apomorphine were concomitantly administered to healthy subjects, the mean largest decrease (the mean of each subject's largest drop in blood pressure measured within the 6-hour period following administration of subcutaneous apomorphine) for supine systolic and diastolic blood pressure was 9.1 mm Hg and 10.5 mm Hg, respectively. The mean largest standing systolic and diastolic blood pressure decrease was 11.3 mm Hg and 12.6 mm Hg, respectively. In some individuals, the decrease was as high as 61 mm Hg and 51 mm Hg, respectively, for standing systolic and diastolic blood pressure.
When low dose ethanol (0.3 g/kg) and subcutaneous apomorphine were concomitantly administered, the mean largest decrease in supine systolic and diastolic blood pressure was 10.2 mm Hg and 9.9 mm Hg, respectively. The mean largest decrease in standing systolic and diastolic blood pressure was 8.4 mm Hg and 7.1 mm Hg, respectively. In comparison, the mean largest decrease in supine systolic and diastolic blood pressure when subcutaneous apomorphine was administered alone was 6.1 mm Hg and 7.3 mm Hg, respectively, and in standing systolic and diastolic blood pressure was 6.7 mm Hg 8.4 mm Hg, respectively.
A similar study has not been performed with KYNMOBI.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lifetime carcinogenicity studies of apomorphine were conducted in male (0.1, 0.3, or 0.8 mg/kg/day) and female (0.3, 0.8, or 2 mg/kg/day) rats. Apomorphine was administered by subcutaneous injection for 22 months or 23 months, respectively. In males, there was an increase in Leydig cell tumors at the highest dose tested. This finding is of questionable significance because the endocrine mechanisms believed to be involved in the production of Leydig cell tumors in rats are not relevant to humans. No drug-related tumors were observed in females.
In a 26-week carcinogenicity study in P53-knockout transgenic mice, there was no evidence of carcinogenic potential when apomorphine was administered by subcutaneous injection at doses up to 20 mg/kg/day (male) or 40 mg/kg/day (female).
Mutagenesis
Apomorphine was mutagenic in the in vitro bacterial reverse mutation (Ames) and the in vitro mouse lymphoma tk assays. Apomorphine was clastogenic in the in vitro chromosomal aberration assay in human lymphocytes and in the in vitro mouse lymphoma tk assay. Apomorphine was negative in the in vivo micronucleus assay in mice.
Impairment of Fertility
Apomorphine was administered subcutaneously at doses up to 3 mg/kg/day to male and female rats prior to and throughout the mating period and continuing in females through gestation day 6. There was no evidence of adverse effects on fertility or on early fetal viability. A significant decrease in testis weight was observed in a 39-week study in cynomolgus monkey at all subcutaneous doses tested (0.3, 1, or 1.5 mg/kg/day).
In a published fertility study, apomorphine was administered to male rats at subcutaneous doses of 0.2, 0.8, or 2 mg/kg prior to and throughout the mating period. Fertility was reduced at the highest dose tested.
-
14 CLINICAL STUDIES
The efficacy of KYNMOBI for the acute, intermittent treatment of “off” episodes in patients with Parkinson's disease was established in one randomized, double-blind, placebo-controlled, parallel-group study (Study 1; NCT02469090).
The study enrolled patients with a mean duration of Parkinson's disease of approximately 9 years (range: 2 years to 22 years) who were Hoehn and Yahr Stage III or less in the “on” state, and who were all receiving concomitant levodopa with a stable dose for at least 4 weeks before screening. The most commonly used concomitant Parkinson's disease medications in addition to levodopa were oral dopaminergic agonists (51%), monoamine oxidase B inhibitors (41%), amantadine derivatives (21%), and other dopaminergic agents (8%).
At baseline, the mean number of daily “off” episodes was 4 and the mean duration of “off” episodes was slightly over an hour in both groups. The study included a titration phase and a 12-week maintenance phase. Patients were titrated to the dose that achieved a full “on” response and was tolerated during the titration phase. Patients were treated with an oral antiemetic starting 3 days before the titration phase. In the titration phase, patients (N=141) arrived at the study site in an “off” state having not taken their regular morning dose of carbidopa/levodopa or any other adjunctive PD medications, as well as having taken their last dose of carbidopa/levodopa and any other adjunctive PD medications no later than midnight the night before. Treatment was initiated in the clinic with a 10 mg dose of KYNMOBI. If the patient responded to treatment and tolerated the 10 mg KYNMOBI dose, the patient was randomized in a blinded fashion to KYNMOBI or placebo in a 1:1 ratio. If the patient tolerated the dose but did not adequately respond, the patient was asked to return to the clinic within 3 days and the dose was increased by 5 mg. The titration process was continued up to a maximum KYNMOBI dose of 35 mg or until a full “on” was achieved as determined by the investigator and the patient [see Dosage and Administration (2.2)]. Dose administration was permitted up to five times per day in the maintenance phase. The Movement Disorder Society-Unified Parkinson's Disease Rating Scale, Part III (MDS-UPDRS III) (motor examination) was measured pre dose, and at 15, 30, 45, 60, and 90 minutes post dose.
The primary endpoint of the study was the mean change from pre dose to 30 minutes post dose in the MDS-UPDRS III at the 12-week visit of the maintenance phase.
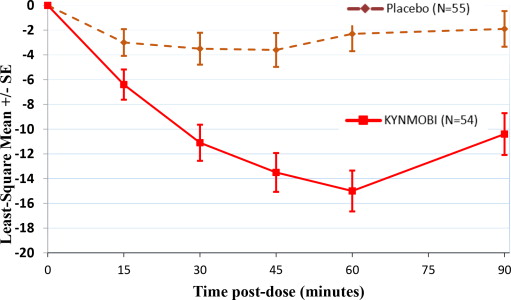
A total of 54 patients were randomized to KYNMOBI and 55 patients to placebo. The KYNMOBI treatment group showed a least-square mean improvement (i.e., reduction in score) of -11.1 points (95% CI: -14.0, -8.2), versus -3.5 points for the placebo group (95% CI: -6.1, -0.9). The least-square mean treatment difference between KYNMOBI and placebo was -7.6 (95% CI: -11.5, -3.7; p = 0.0002) (Table 2).
Table 2: Change from Pre-dose to 30 Minutes Post-dose in the MDS-UPDRS III Score at Week 12 (Least-Square Mean) in Study 1 Treatment Number of Patients at Week 12 Observed Pre-dose MDS-UPDRS III Score at Week 12 Least-Square Mean Change from Pre-dose to 30 Minutes Post-dose Least-Square Mean Difference from placebo Placebo 46 42.2 - 3.5 N/A KYNMOBI 34 37.2 - 11.1 - 7.6 (p=0.0002) Figure 2 describes the least-square mean change from pre-dose in MDS-UPDRS Part III Motor Scores after administration of KYNMOBI versus placebo at week 12.
Figure 2: Estimated Least-Square Mean Change in MDS-UPDRS Part III Motor Score After Administration of KYNMOBI vs. Placebo (at Week 12) in Study 1
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
KYNMOBI sublingual film is a blue to green rectangular film with a white printed number identifying the strength (e.g., “10” is 10 mg). Each sublingual film is individually packaged in a sealed foil pouch. Films are supplied in the following strengths and package configurations (Table 3):
Table 3: Package Configuration for KYNMOBI sublingual film Single Film Strength
(NDC Code)Package Configuration NDC Code Trade Titration Kit
10 mg (63402-010-01)
15 mg (63402-015-01)
20 mg (63402-020-01)
25 mg (63402-025-01)
30 mg (63402-030-01)Each titration kit carton will contain a total of 10 individually packaged films of:
2 – single 10 mg films
2 – single 15 mg films
2 – single 20 mg films
2 – single 25 mg films
2 – single 30 mg films63402-088-10 Trade Product 10 mg (63402-010-01) 30 films per carton 63402-010-30 15 mg (63402-015-01) 30 films per carton 63402-015-30 20 mg (63402-020-01) 30 films per carton 63402-020-30 25 mg (63402-025-01) 30 films per carton 63402-025-30 30 mg (63402-030-01) 30 films per carton 63402-030-30 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Administration of KYNMOBI
Advise patients that KYNMOBI is for sublingual use only [see Dosage and Administration (2.1)].
KYNMOBI must be administered whole. Advise patients not to cut, chew, or swallow KYNMOBI.
Nausea and Vomiting
Advise patients that KYNMOBI may cause nausea and vomiting when it is administered at recommended doses. Treatment with an antiemetic (e.g., trimethobenzamide) may be used, as needed, if nausea or vomiting occurs. Inform patients that they should discuss with their healthcare provider when the antiemetic can be discontinued [see Warnings and Precautions (5.1)].
Falling Asleep During Activities of Daily Living and Somnolence
Alert patients to the potential sedating effects of KYNMOBI, including somnolence and falling asleep while engaged in activities of daily living. Instruct patients not to drive a car or engage in other potentially dangerous activities until they have gained sufficient experience with KYNMOBI to gauge whether or not it affects their mental and/or motor performance adversely. Advise patients that if increased somnolence or episodes of falling asleep during activities of daily living (e.g., watching television, passenger in a car, etc.) occur, they should not drive or participate in potentially dangerous activities until they have discussed this with their healthcare provider. Because of possible additive effects of alcohol use, advise patients to limit their alcohol intake [see Warnings and Precautions (5.2)].
Hypersensitivity / Allergic Reactions
Advise patients that hypersensitivity/allergic reaction (e.g., swelling of the lips, tongue and mouth, flushing, and, infrequently, urticaria and throat tightness) may occur because of apomorphine, sodium metabisulfite, or any KYNMOBI excipients. Inform patients with a sulfite sensitivity that KYNMOBI contains sodium metabisulfite, a sulfite that may cause allergic-type reactions, including anaphylactic symptoms and life-threatening or less severe asthmatic episodes [see Warnings and Precautions (5.3)]. Advise patients who experience any hypersensitivity/allergic reaction to KYNMOBI that they should avoid taking KYNMOBI again [see Contraindications (4)].
Hypotension/Orthostatic Hypotension
Advise patients that they may develop postural (orthostatic) hypotension with or without symptoms, such as dizziness, nausea, syncope, and sweating. Instruct patients to rise slowly after sitting or lying down after taking KYNMOBI. Instruct patients to limit their alcohol intake because it may potentiate the hypotensive effect of KYNMOBI. Instruct patients to lie down before and after taking sublingual nitroglycerin because it may potentiate the hypotensive effect of KYNMOBI [see Warnings and Precautions (5.4)].
Oral Mucosal Irritation
Inform patients that KYNMOBI may result in oral mucosal adverse reactions such as irritation, erythema, lip swelling, mouth ulceration, dry mouth, stomatitis, glossodynia, oropharyngeal pain, swollen tongue, ageusia, oral pain, lip ulceration, oral disorder and hypoaesthesia oral [see Warnings and Precautions (5.5) and Adverse Reactions (6.1)].
Falls
Alert patients that they may have increased risk for falling when using KYNMOBI [see Warnings and Precautions (5.6)].
Hallucinations and/or Psychotic-Like Behavior
Inform patients that KYNMOBI may cause hallucinations or other manifestations of psychotic-like behavior. Advise patients to inform their healthcare provider if they have a major psychotic disorder or are taking any treatments for psychosis [see Warnings and Precautions (5.7)].
Impulse Control / Compulsive Behaviors
Patients and their caregivers should be alerted to the possibility that they may experience intense urges to spend money uncontrollably, intense urges to gamble, increased sexual urges, binge eating and/or other intense urges and the inability to control these urges while taking KYNMOBI [see Warnings and Precautions (5.8)].
Withdrawal-Emergent Hyperpyrexia and Confusion
Advise patients to contact their healthcare provider if they wish to discontinue KYNMOBI or decrease the dose of KYNMOBI [see Warnings and Precautions (5.9)].
QTc Prolongation and Potential for Proarrhythmic Effects
Alert patients that KYNMOBI may cause QTc prolongation and might produce proarrhythmic effects that could cause torsades de pointes and sudden death. Palpitations and syncope may signal the occurrence of an episode of torsades de pointes [see Warnings and Precautions (5.10)].
Priapism
Advise patients that KYNMOBI may cause prolonged painful erections and that if this occurs that they should seek medical attention immediately [see Warnings and Precautions (5.12)].
Manufactured for:
Sunovion Pharmaceuticals Inc.
Marlborough, Massachusetts 01752 USAKYNMOBI is a trademark of Sunovion Pharmaceuticals Inc.
SUNOVION and are registered trademarks of Sumitomo Dainippon Pharma Co. Ltd.
Sunovion Pharmaceuticals Inc. is a U.S. subsidiary of Sumitomo Dainippon Pharma Co. Ltd.
©2020 Sunovion Pharmaceuticals Inc. All rights reserved.
For more information, go to www.KYNMOBI.com or call Sunovion Customer Service at 1-888-394-7377.10413-01
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration Issued: 5/2020
Patient Information
KYNMOBI™ (kin-moe′-bee)
(apomorphine hydrochloride)
sublingual filmRead this Patient Information before you start taking KYNMOBI and each time you get a refill. There may be new information. This Patient Information does not take the place of talking to your healthcare provider about your medical condition or treatment. What is KYNMOBI?
KYNMOBI is a prescription medicine used to treat short-term (acute), intermittent “off” episodes in people with Parkinson's disease (PD).
It is not known if KYNMOBI is safe and effective in children.Do not take KYNMOBI if you are:
- taking certain medicines to treat nausea called 5HT3 antagonists including ondansetron, granisetron, dolasetron, palonosetron, and alosetron. People taking ondansetron together with apomorphine, the active ingredient in KYNMOBI, have had very low blood pressure and lost consciousness or “blacked out.”
- allergic to apomorphine hydrochloride or to any of the ingredients in KYNMOBI. See the end of the Patient Information leaflet for a complete list of ingredients in KYNMOBI.
KYNMOBI also contains a sulfite called sodium metabisulfite. Sulfites can cause severe, life-threatening allergic reactions in some people. An allergy to sulfites is not the same as an allergy to sulfa. People with asthma are more likely to be allergic to sulfites.
Call your healthcare provider or get emergency help right away if you get any of the following symptoms of a severe life-threatening allergic reaction:
- hives
- itching
- rash
- swelling of the lips, tongue, and mouth
- redness of your face (flushing)
- throat tightness
- trouble breathing or swallowing
Before you start taking KYNMOBI, tell your healthcare provider about all of your medical conditions, including if you:
- have difficulty staying awake during the daytime.
- have dizziness.
- have fainting spells.
- have low blood pressure.
- have asthma.
- are allergic to any medicines containing sulfites
- have liver problems.
- have kidney problems.
- have heart problems.
- have had a stroke or other brain problems.
- have a mental problem called a major psychotic disorder.
- drink alcohol.
- are pregnant or plan to become pregnant. It is not known if KYNMOBI will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if KYNMOBI passes into your breast milk. You and your healthcare provider should decide if you will take KYNMOBI or breastfeed.
- Tell your healthcare provider about all the medicines you take, including prescription medicines and, over-the-counter medicines, vitamins, and herbal supplements.
KYNMOBI may affect the way other medicines work, and other medicines can affect how KYNMOBI works.
Taking KYNMOBI with other medicines may cause serious side effects.
- If you take nitroglycerin under your tongue (sublingual) while using KYNMOBI, your blood pressure may decrease and cause dizziness. You should lie down before and after taking sublingual nitroglycerin.
Know the medicines you take. Keep a list of your medicines with you and show it to your healthcare provider and pharmacist when you get a new medicine. How should I take KYNMOBI?
- Read the step-by-step Instructions for Use that comes with your KYNMOBI prescription.
- Take KYNMOBI exactly as your healthcare provider tells you to take it.
- Your healthcare provider will tell you how much KYNMOBI to take and teach you the right way to take it.
- Your healthcare provider may change your dose if needed.
- Do not change your dose of KYNMOBI or take it more often than prescribed unless your healthcare provider has told you to.
- Do not take more than 1 dose of KYNMOBI to treat a “OFF” episode.
- Do not take another dose of KYNMOBI sooner than 2 hours after the last dose.
- Do not take KYNMOBI more than 5 times a day.
- Do not cut, chew or swallow KYNMOBI.
- Your healthcare provider may prescribe another medicine for nausea called an antiemetic to take while you are taking KYNMOBI. Antiemetic medicines help to decrease the symptoms of nausea and vomiting that can happen when you take KYNMOBI.
What should I avoid while taking KYNMOBI?
- Do not drink alcohol while you are taking KYNMOBI. It can increase your chance of developing serious side effects.
- Do not take medicines that make you sleepy while you are using KYNMOBI.
- Do not drive, operate machinery, or do other dangerous activities until you know how KYNMOBI affects you.
- Do not change your body position too fast. Get up slowly from sitting or lying. KYNMOBI can lower your blood pressure and cause dizziness or fainting.
What are the possible side effects of KYNMOBI?
KYNMOBI can cause serious side effects, including:
- nausea and vomiting. Nausea is a common side effect of KYNMOBI. Nausea and vomiting can happen with KYNMOBI. Your healthcare provider may prescribe a medicine called an antiemetic, such as trimethobenzamide, to help prevent nausea and vomiting. Some patients can stop taking trimethobenzamide after using KYNMOBI, when advised by your healthcare provider. Some patients may need to keep taking trimethobenzamide to help treat nausea and vomiting. Talk to your healthcare provider before you stop taking trimethobenzamide.
- sleepiness or falling asleep during the day. Sleepiness is a serious and common side effect of KYNMOBI. Some people treated with KYNMOBI may get sleepy during the day or fall asleep without warning while doing everyday activities such as talking, eating, or driving a car.
- allergic reactions. See the “Do not take KYNMOBI if you are” section.
- dizziness. Dizziness is a serious, and common side effect of KYNMOBI. KYNMOBI may lower your blood pressure and cause dizziness. Dizziness can happen when KYNMOBI treatment is started or when the KYNMOBI dose is increased. Do not get up too fast from sitting or after lying down, especially if you have been sitting or lying down for a long period of time.
- mouth (oral) irritation. Mouth (oral) irritation is a common side effect of KYNMOBI. You should call your healthcare provider if you develop any of these signs or symptoms:
- redness
- swelling
- mouth sores (ulceration)
- pain
- dryness of the mouth, lips or tongue
- pain with swallowing
These signs and symptoms may go away if KYNMOBI treatment is stopped.
- falls. The changes that can happen with PD, and the effects of some PD medicines, can increase the risk of falling. KYNMOBI may also increase your risk of falling.
- hallucinations or psychotic-like behavior. KYNMOBI may cause or make psychotic-like behavior worse including hallucinations (seeing or hearing things that are not real), confusion, excessive suspicion, aggressive behavior, agitation, delusional beliefs (believing things that are not real), and disorganized thinking.
- strong (intense) urges. Some people with PD have reported new or strong uncontrollable urges to gamble, increased sexual urges, increased urges to spend money (compulsive shopping), and other intense urges, while taking PD medicines, including KYNMOBI. If you or your family members notice that you have strong urges, talk to your healthcare provider. The strong urges may go away if your KYNMOBI dose is lowered or stopped.
- high fever and confusion. KYNMOBI may cause a problem that can happen in people who suddenly lower their dose, stop using, or change their dose of KYNMOBI. Symptoms include:
- very high fever
- stiff muscles
- confusion
- changes in breathing and heartbeat
Do not stop taking KYNMOBI or change your dose unless you are told to do so by your healthcare provider.
- heart problems. If you have shortness of breath, fast heartbeat, chest pain, or feel like you are going to pass out (faint) while taking KYNMOBI, call your healthcare provider or get emergency help right away.
- tissue changes (fibrotic complications). Some people have had changes in the tissues of their pelvis, lungs, and heart valves when taking medicines called nonergot derived dopamine agonists like KYNMOBI.
-
prolonged painful erections (priaprism). KYNMOBI may cause prolonged, painful erections in some people. If you have a prolonged and painful erection you should call your healthcare provider or go to the nearest hospital emergency room right away.
If you have any of these symptoms, stop taking KYNMOBI and call your healthcare provider right away before
taking another dose.
The most common side effects of KYNMOBI include:- nausea
- sleepiness
- dizziness
- mouth swelling, pain, or sores
These are not all of the possible side effects of KYNMOBI.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store KYNMOBI?
- Store KYNMOBI at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep KYNMOBI in the foil pouch until you are ready to take it.
Keep KYNMOBI and all medicines out of the reach of children. General information about the safe and effective use of KYNMOBI.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use KYNMOBI for a condition for which it was not prescribed. Do not give KYNMOBI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about KYNMOBI that is written for health professionals.What are the ingredients in KYNMOBI?
Active ingredient: apomorphine hydrochloride
Inactive ingredients: disodium EDTA, dihydrate, FD&C Blue #1, glycerol, glyceryl monostearate, hydroxyethyl cellulose, hypromellose, maltodextrin, (-)-menthol, pyridoxine hydrochloride, sodium hydroxide, sodium metabisulfite, sucralose, and white ink.

Manufactured for:
Sunovion Pharmaceuticals Inc.
Marlborough, Massachusetts 01752 USA
KYNMOBI is a trademark of Sunovion Pharmaceuticals Inc.
SUNOVION and are registered trademarks of Sumitomo Dainippon Pharma Co. Ltd.
Sunovion Pharmaceuticals Inc. is a U.S. subsidiary of Sumitomo Dainippon Pharma Co. Ltd.
©2020 Sunovion Pharmaceuticals Inc. All rights reserved.
For more information, go to www.KYNMOBI.com or call Sunovion Customer Service at 1-888-394-7377.
10413-01 -
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
KYNMOBITM (kin-moe′-bee)
(apomorphine hydrochloride)
Sublingual FilmRead this Instructions for Use before you start taking KYNMOBI and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment. Important
- KYNMOBI is for sublingual (under your tongue) use only.
- KYNMOBI must be taken whole. Do not cut, chew, or swallow KYNMOBI.
- Do not take KYNMOBI until you talk with your healthcare provider about how to take it.
- Check the expiration date printed on the foil pouch. Do not use KYNMOBI if the expiration date has passed.
- Do not take more than 1 dose of KYNMOBI every 2 hours.
- Do not take more than 5 doses of KYNMOBI each day.
How to store KYNMOBI - Store KYNMOBI at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep KYNMOBI in the foil pouch until you are ready to take it.
- Keep KYNMOBI and all medicines out of the reach of children.
How KYNMOBI is packaged
Inside each child-resistant carton is a plastic tray with a pull-out handle that holds the sealed pouches of KYNMOBI sublingual film (see Figure A). Each KYNMOBI sublingual film comes in a sealed foil pouch (see Figure B).Figure A

Figure B
ATTENTION: Read the Instructions for Use on the other side of this leaflet. -
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE Instructions on How to Use the Child-Resistant Carton STEP 1 Open Carton
Open carton from the end with the arrow. Push in both tabs to unlock. Hold tabs in. (See Figure C.)
Lift up tray handle. Pull out tray (see Figure D).STEP 2 Remove Pouch
Push finger up through the hole in the bottom of tray. (See Figure E.)
Firmly pull one (1) pouch from the tray (see Figure F).STEP 3 Close Carton
To close, slide tray in until it clicks. (See Figure G.)
This ensures the carton remains child resistant (see Figure H).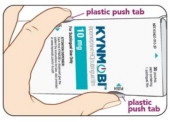
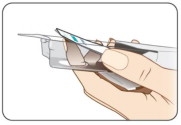
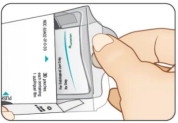
Figure C Figure E Figure G 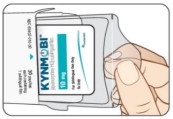

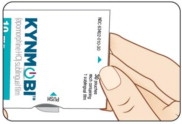
Figure D Figure F Figure H Instructions for Taking KYNMOBI Step 4
- Your healthcare provider has told you to take KYNMOBI 10 mg, 15 mg, 20 mg, 25 mg, or 30 mg. Complete Steps 5 through 10 to take KYNMOBI.
Step 5
Drink water. Before taking each KYNMOBI, drink water to moisten your mouth.
This helps the film dissolve more easily (see Figure I).
Step 6
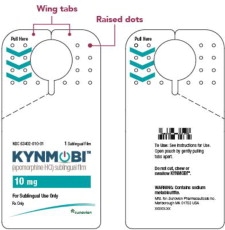
Open the KYNMOBI foil pouch.
Hold the wing tabs on the pouch between your thumb and pointer finger of each hand. Make sure to place your fingers directly on the raised dots on each wing tab.
Gently pull the wing tabs apart to open the pouch
(see Figure J).Step 7
Take KYNMOBI out of the pouch.
Hold KYNMOBI between your fingers by the outside edges and remove the entire KYNMOBI from the pouch (see Figure K).
KYNMOBI must be taken whole. Throw away KYNMOBI if it is broken or missing pieces. Use a new KYNMOBI for your dose.
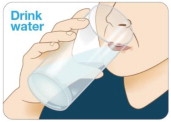 Figure I
Figure I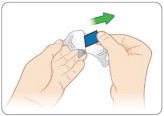 Figure K
Figure K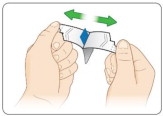 Figure J
Figure JStep 8
Place entire KYNMOBI under your tongue. Place KYNMOBI as far back under your tongue as you can (see Figure L).
Close your mouth.
Step 9
Keep KYNMOBI in place until it has completely dissolved (see Figure M).
- Do not chew or swallow KYNMOBI.
-
Do not swallow your saliva or talk while KYNMOBI is dissolving because this can affect how well the medicine in KYNMOBI is absorbed.
Step 10
Open your mouth to check if KYNMOBI has completely dissolved.
It can take about 3 minutes for KYNMOBI to dissolve.
After the film completely dissolves, you may swallow.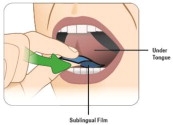 Figure L
Figure L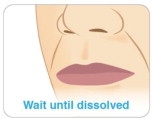 Figure M
Figure MFor assistance with the KYNMOBI child-resistant carton, please ask your care partner for help.
You may also contact your doctor or Sunovion Customer Service at 1-888-394-7377 with questions or for support.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.Manufactured for Sunovion Pharmaceuticals Inc. Marlborough, Massachusetts 01752 USA.
KYNMOBI and are trademarks of Sunovion Pharmaceuticals Inc.
are trademarks of Sunovion Pharmaceuticals Inc.
SUNOVION and are registered trademarks of Sumitomo Dainippon Pharma Co. Ltd. Sunovion Pharmaceuticals Inc. is a U.S. subsidiary of Sumitomo Dainippon Pharma Co. Ltd.
are registered trademarks of Sumitomo Dainippon Pharma Co. Ltd. Sunovion Pharmaceuticals Inc. is a U.S. subsidiary of Sumitomo Dainippon Pharma Co. Ltd.
©2020 Sunovion Pharmaceuticals Inc. All rights reserved.
Issued 5/2020
10414-01(See reverse side for additional information) - Your healthcare provider has told you to take KYNMOBI 10 mg, 15 mg, 20 mg, 25 mg, or 30 mg. Complete Steps 5 through 10 to take KYNMOBI.
-
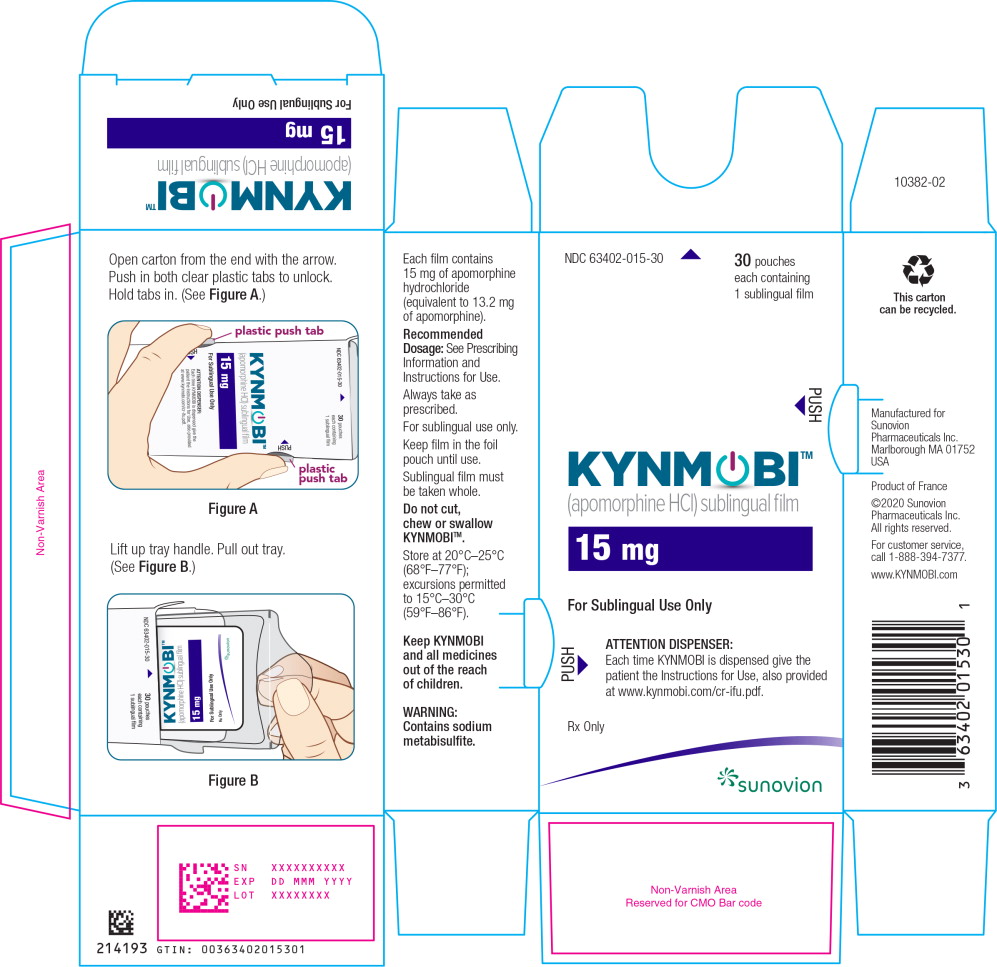
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – TRADE POUCH LABEL – 10 mg
NDC: 63402-010-01 1 sublingual film
Apomorphine HCl
sublingual film
10 mg
For Sublingual Use Only
Rx only
SUNOVION
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – TRADE POUCH LABEL – 15 mg
NDC: 63402-015-01 1 sublingual film
Apomorphine HCl
sublingual film
15 mg
For Sublingual Use Only
Rx only
SUNOVION
-
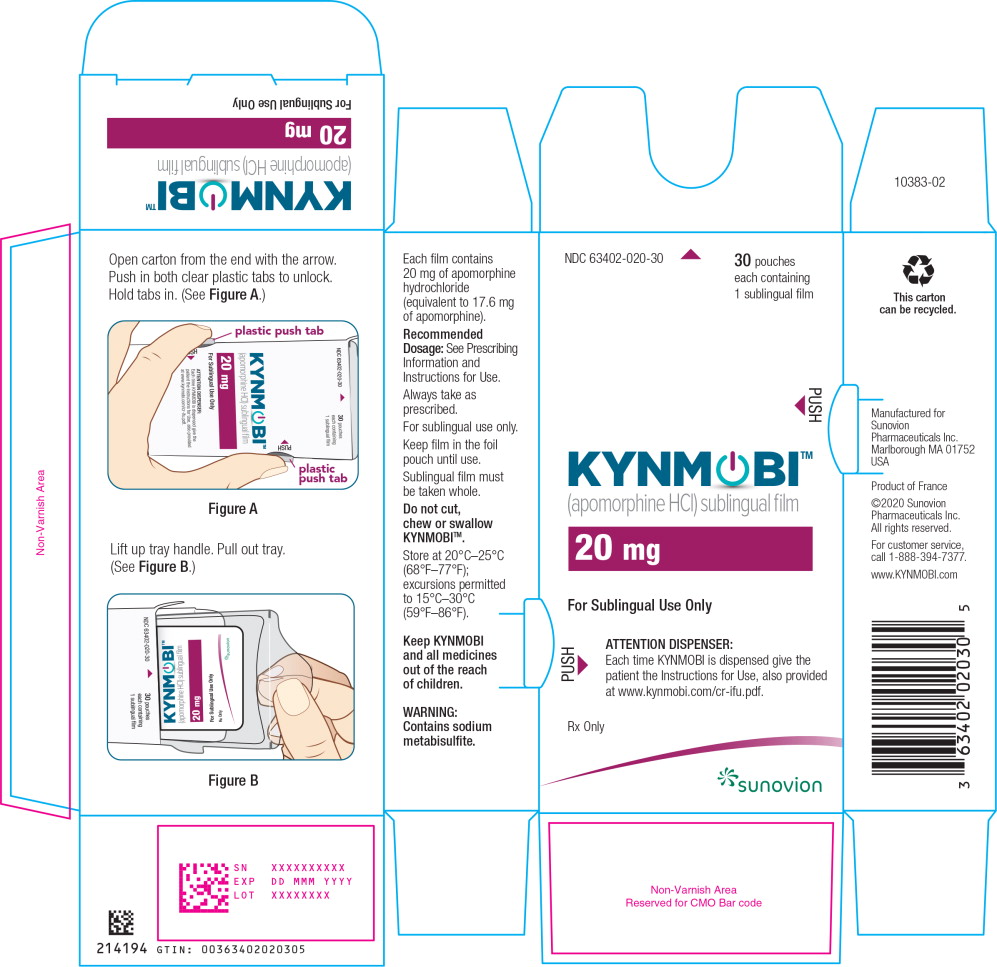
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – TRADE POUCH LABEL – 20 mg
NDC: 63402-020-01 1 sublingual film
Apomorphine HCl
sublingual film
20 mg
For Sublingual Use Only
Rx only
SUNOVION
-
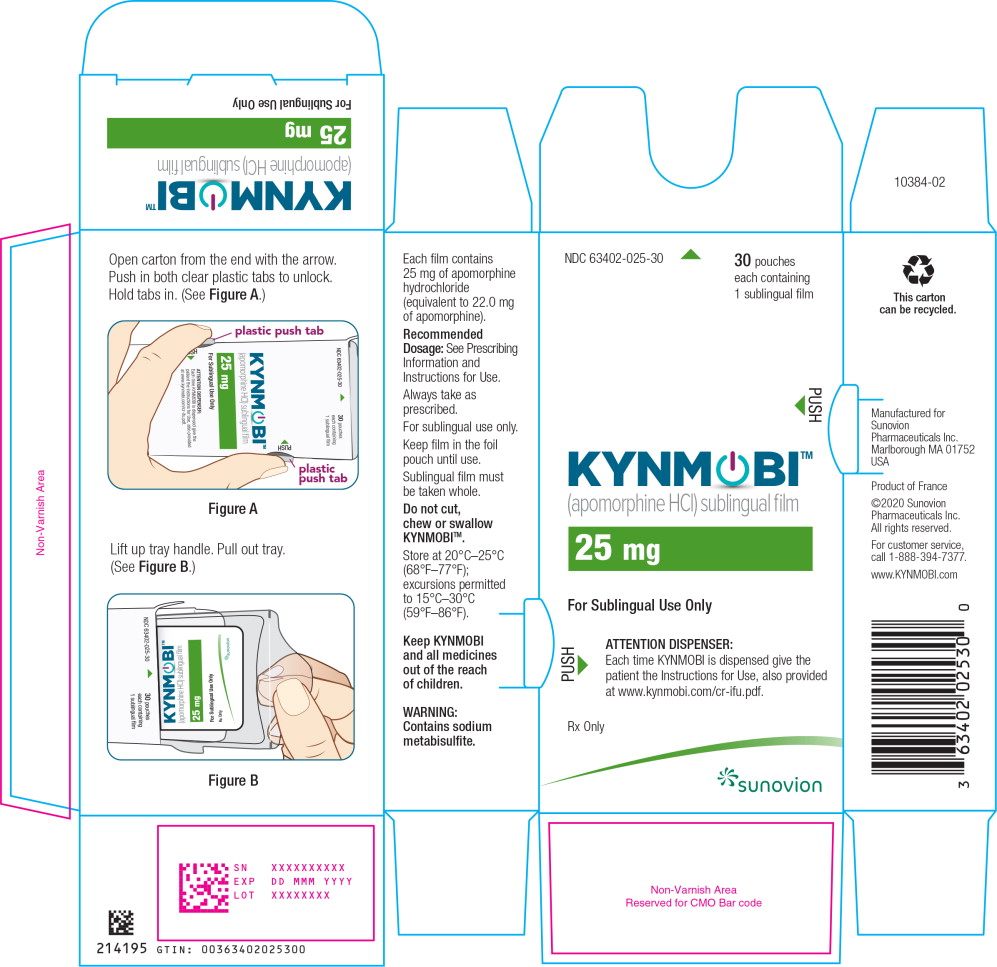
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – TRADE POUCH LABEL – 25 mg
NDC: 63402-025-01 1 sublingual film
Apomorphine HCl
sublingual film
25 mg
For Sublingual Use Only
Rx only
SUNOVION
-
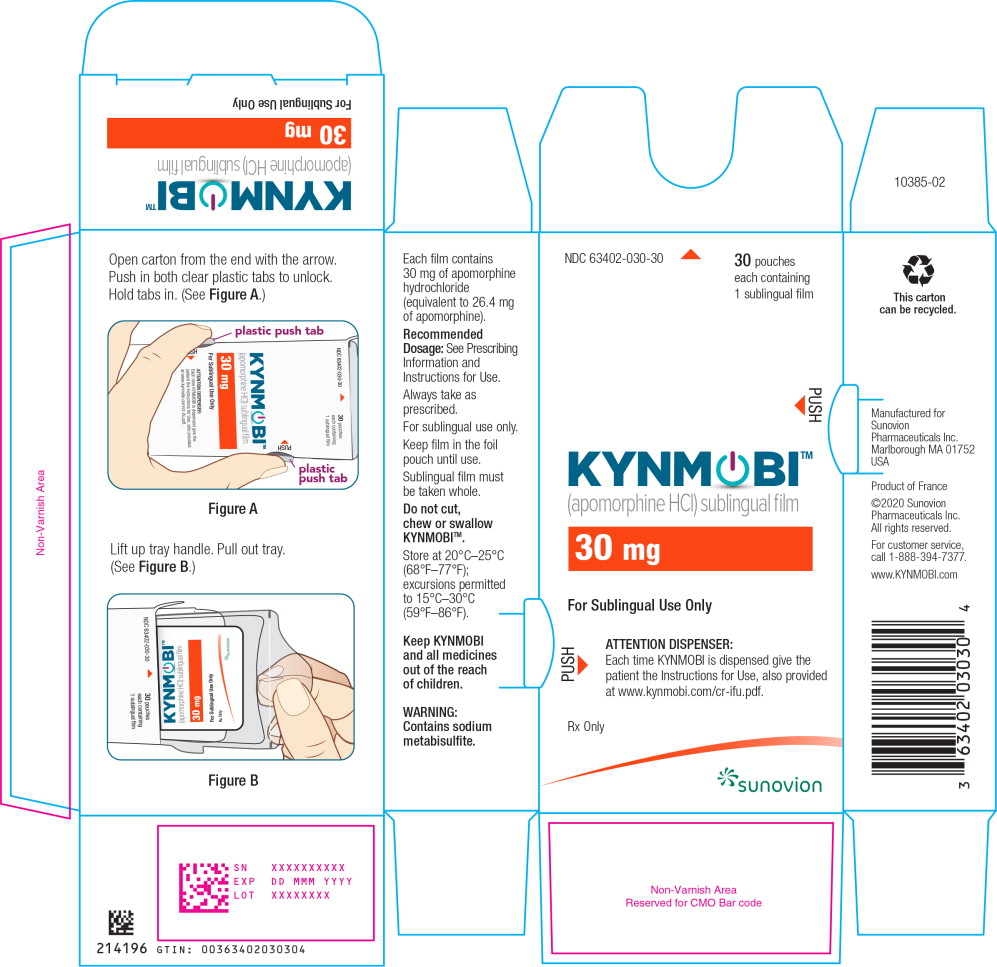
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – TRADE POUCH LABEL – 30 mg
NDC: 63402-030-01 1 sublingual film
Apomorphine HCl
sublingual film
30 mg
For Sublingual Use Only
Rx only
SUNOVION
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – TRADE CARTON – 10 mg
NDC: 63402-010-30 30 pouches each containing 1 sublingual film
KYNMOBI™
(apomorphine HCl) sublingual film
10 mg
For Sublingual Use Only
ATTENTION DISPENSER:
Each time KYNMOBI is dispensed give the patient the Instructions for Use, also provided at www.kynmobi.com/cr-ifu.pdf
Rx only
SUNOVION
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – TRADE CARTON – 15 mg
NDC: 63402-015-30 30 pouches each containing 1 sublingual film
KYNMOBI™
(apomorphine HCl) sublingual film
15 mg
For Sublingual Use Only
ATTENTION DISPENSER:
Each time KYNMOBI is dispensed give the patient the Instructions for Use, also provided at www.kynmobi.com/cr-ifu.pdf
Rx only
SUNOVION
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – TRADE CARTON – 20 mg
NDC: 63402-020-30 30 pouches each containing 1 sublingual film
KYNMOBI™
(apomorphine HCl) sublingual film
20 mg
For Sublingual Use Only
ATTENTION DISPENSER:
Each time KYNMOBI is dispensed give the patient the Instructions for Use, also provided at www.kynmobi.com/cr-ifu.pdf
Rx only
SUNOVION
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – TRADE CARTON – 25 mg
NDC: 63402-025-30 30 pouches each containing 1 sublingual film
KYNMOBI™
(apomorphine HCl) sublingual film
25 mg
For Sublingual Use Only
ATTENTION DISPENSER:
Each time KYNMOBI is dispensed give the patient the Instructions for Use, also provided at www.kynmobi.com/cr-ifu.pdf
Rx only
SUNOVION
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – TRADE CARTON – 30 mg
NDC: 63402-030-30 30 pouches each containing 1 sublingual film
KYNMOBI™
(apomorphine HCl) sublingual film
30 mg
For Sublingual Use Only
ATTENTION DISPENSER:
Each time KYNMOBI is dispensed give the patient the Instructions for Use, also provided at www.kynmobi.com/cr-ifu.pdf
Rx only
SUNOVION
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – TRADE OUTER TITRATION CARTON – 10 mg, 15 mg, 20 mg, 25 mg, 30 mg
NDC: 63402-088-10 10 pouches each containing 1 sublingual film
KYNMOBI™
(apomorphine HCl) sublingual film
10 mg 15 mg 20 mg 25 mg 30 mg
For Sublingual Use Only
Rx only
READ THE INSTRUCTIONS INSIDE THE TOP PANEL AND READ THE COMPLETE INSTRUCTIONS FOR USE BEFORE TAKING KYNMOBITM
SUNOVION
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – SAMPLE TITRATION CARTON – 10 mg, 15 mg, 20 mg, 25 mg, 30 mg
NDC: 63402-188-10 10 pouches each containing 1 sublingual film
PROFESSIONAL SAMPLE – NOT FOR SALE OR REIMBURSEMENT
KYNMOBI™
(apomorphine HCl) sublingual film
10 mg 15 mg 20 mg 25 mg 30 mg
For Sublingual Use Only
Rx only
READ THE INSTRUCTIONS INSIDE THE TOP PANEL AND READ THE COMPLETE INSTRUCTIONS FOR USE BEFORE TAKING KYNMOBITM
CONTENTS OF THIS KIT:
KYNMOBI 10 mg: 2 pouches, each containing 1 sublingual film
KYNMOBI 15 mg: 2 pouches, each containing 1 sublingual film
KYNMOBI 20 mg: 2 pouches, each containing 1 sublingual film
KYNMOBI 25 mg: 2 pouches, each containing 1 sublingual film
KYNMOBI 30 mg: 2 pouches, each containing 1 sublingual film
SUNOVION
-
INGREDIENTS AND APPEARANCE
KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63402-010 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 10 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-010-30 30 in 1 CARTON 05/21/2020 1 NDC: 63402-010-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63402-110 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 10 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-110-30 30 in 1 CARTON 05/21/2020 1 NDC: 63402-110-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63402-015 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 15 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-015-30 30 in 1 CARTON 05/21/2020 1 NDC: 63402-015-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63402-115 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 15 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-115-30 30 in 1 CARTON 05/21/2020 1 NDC: 63402-115-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63402-020 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 20 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-020-30 30 in 1 CARTON 05/21/2020 1 NDC: 63402-020-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63402-120 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 20 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-120-30 30 in 1 CARTON 05/21/2020 1 NDC: 63402-120-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63402-025 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 25 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-025-30 30 in 1 CARTON 05/21/2020 1 NDC: 63402-025-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63402-125 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 25 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-125-30 30 in 1 CARTON 05/21/2020 1 NDC: 63402-125-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63402-030 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 30 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 26mm Flavor Imprint Code 30 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-030-30 30 in 1 CARTON 05/21/2020 1 NDC: 63402-030-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63402-130 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 30 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 26mm Flavor Imprint Code 30 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-130-30 30 in 1 CARTON 05/21/2020 1 NDC: 63402-130-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 KYNMOBI
apomorphine hydrochloride kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63402-088 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-088-10 1 in 1 CARTON; Type 0: Not a Combination Product 05/21/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 POUCH 2 Part 2 2 POUCH 2 Part 3 2 POUCH 2 Part 4 2 POUCH 2 Part 5 2 POUCH 2 Part 1 of 5 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Item Code (Source) NDC: 63402-010 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 10 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-010-02 2 in 1 CARTON 1 NDC: 63402-010-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 Part 2 of 5 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Item Code (Source) NDC: 63402-015 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 15 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-015-02 2 in 1 CARTON 1 NDC: 63402-015-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 Part 3 of 5 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Item Code (Source) NDC: 63402-020 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 20 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-020-02 2 in 1 CARTON 1 NDC: 63402-020-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 Part 4 of 5 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Item Code (Source) NDC: 63402-025 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 25 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-025-02 2 in 1 CARTON 1 NDC: 63402-025-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 Part 5 of 5 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Item Code (Source) NDC: 63402-030 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 30 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 26mm Flavor Imprint Code 30 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-030-02 2 in 1 CARTON 1 NDC: 63402-030-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 KYNMOBI
apomorphine hydrochloride kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63402-188 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-188-10 1 in 1 CARTON; Type 0: Not a Combination Product 05/21/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 POUCH 2 Part 2 2 POUCH 2 Part 3 2 POUCH 2 Part 4 2 POUCH 2 Part 5 2 POUCH 2 Part 1 of 5 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Item Code (Source) NDC: 63402-110 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 10 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-110-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 Part 2 of 5 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Item Code (Source) NDC: 63402-115 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 15 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-115-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 Part 3 of 5 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Item Code (Source) NDC: 63402-120 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 20 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-120-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 Part 4 of 5 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Item Code (Source) NDC: 63402-125 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 25 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 22mm Flavor Imprint Code 25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-125-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 Part 5 of 5 KYNMOBI
apomorphine hydrochloride film, solubleProduct Information Item Code (Source) NDC: 63402-130 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength apomorphine hydrochloride (UNII: F39049Y068) (apomorphine - UNII:N21FAR7B4S) apomorphine hydrochloride 30 mg Inactive Ingredients Ingredient Name Strength Pyridoxine hydrochloride (UNII: 68Y4CF58BV) sodium hydroxide (UNII: 55X04QC32I) sodium metabisulfite (UNII: 4VON5FNS3C) edetate disodium (UNII: 7FLD91C86K) menthol (UNII: L7T10EIP3A) glyceryl monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) maltodextrin (UNII: 7CVR7L4A2D) Sucralose (UNII: 96K6UQ3ZD4) Hydroxyethyl cellulose (280 MPA.S AT 2%) (UNII: 12VCE9HR9E) hydroxyethyl cellulose (140 MPA.S AT 5%) (UNII: 8136Y38GY5) hydroxypropyl cellulose (45000 wamw) (UNII: 8VAB711C5E) FD&C Blue no. 1 (UNII: H3R47K3TBD) water (UNII: 059QF0KO0R) acetone (UNII: 1364PS73AF) alcohol (UNII: 3K9958V90M) Product Characteristics Color blue (blue) Score no score Shape RECTANGLE (RECTANGLE) Size 26mm Flavor Imprint Code 30 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63402-130-01 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210875 05/21/2020 Labeler - Sunovion Pharmaceuticals Inc. (131661746)
Trademark Results [KYNMOBI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KYNMOBI 88319635 not registered Live/Pending |
Sunovion Pharmaceuticals Inc. 2019-02-28 |
 KYNMOBI 87765645 not registered Live/Pending |
Sunovion Pharmaceuticals Inc. 2018-01-23 |
 KYNMOBI 86656477 not registered Dead/Abandoned |
SUNOVION PHARMACEUTICALS INC. 2015-06-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.