HISTEX PDX Drops by Allegis Pharmaceuticals, LLC HISTEX™ PDX Drops
HISTEX PDX Drops by
Drug Labeling and Warnings
HISTEX PDX Drops by is a Otc medication manufactured, distributed, or labeled by Allegis Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HISTEX PDX DROPS- triprolidine hydrochloride syrup
Allegis Pharmaceuticals, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
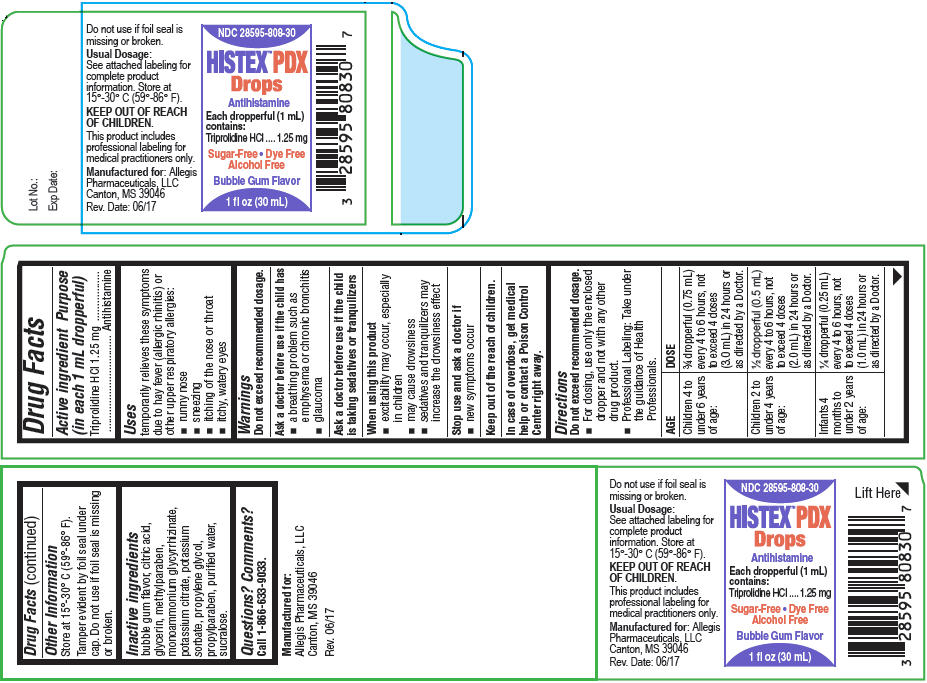
HISTEX™ PDX Drops
Uses
temporarily relieves these symptoms due to hay fever (allergic rhinitis) or other upper respiratory allergies:
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
Warnings
Ask a doctor before use if the child has
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
Directions
Do not exceed recommended dosage.
- For dosing, use only the enclosed dropper and not with any other drug product.
- Professional Labeling: Take under the guidance of Health Professionals.
| AGE | DOSE |
|---|---|
| Children 4 to under 6 years of age: | ¾ dropperful (0.75 mL) every 4 to 6 hours, not to exceed 4 doses (3.0 mL) in 24 hours or as directed by a Doctor. |
| Children 2 to under 4 years of age: | ½ dropperful (0.5 mL) every 4 to 6 hours, not to exceed 4 doses (2.0 mL) in 24 hours or as directed by a Doctor. |
| Infants 4 months to under 2 years of age: | ¼ dropperful (0.25 mL) every 4 to 6 hours, not to exceed 4 doses (1.0 mL) in 24 hours or as directed by a Doctor. |
Other Information
Store at 15°-30° C (59°-86° F).
Tamper evident by foil seal under cap. Do not use if foil seal is missing or broken.
Inactive ingredients
bubble gum flavor, citric acid, glycerin, methylparaben, monoammonium glycyrrhizinate, potassium citrate, potassium sorbate, propylene glycol, propylparaben, purified water, sucralose.
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton
NDC: 28595-808-30
HISTEX™ PDX
Drops
Antihistamine
Each dropperful (1 mL)
contains:
Triprolidine HCl
1.25 mg
Sugar-Free Dye Free
Alcohol Free
Bubble Gum Flavor
1 fl oz (30 mL)
| HISTEX PDX DROPS
triprolidine hydrochloride syrup |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Allegis Pharmaceuticals, LLC (792272861) |