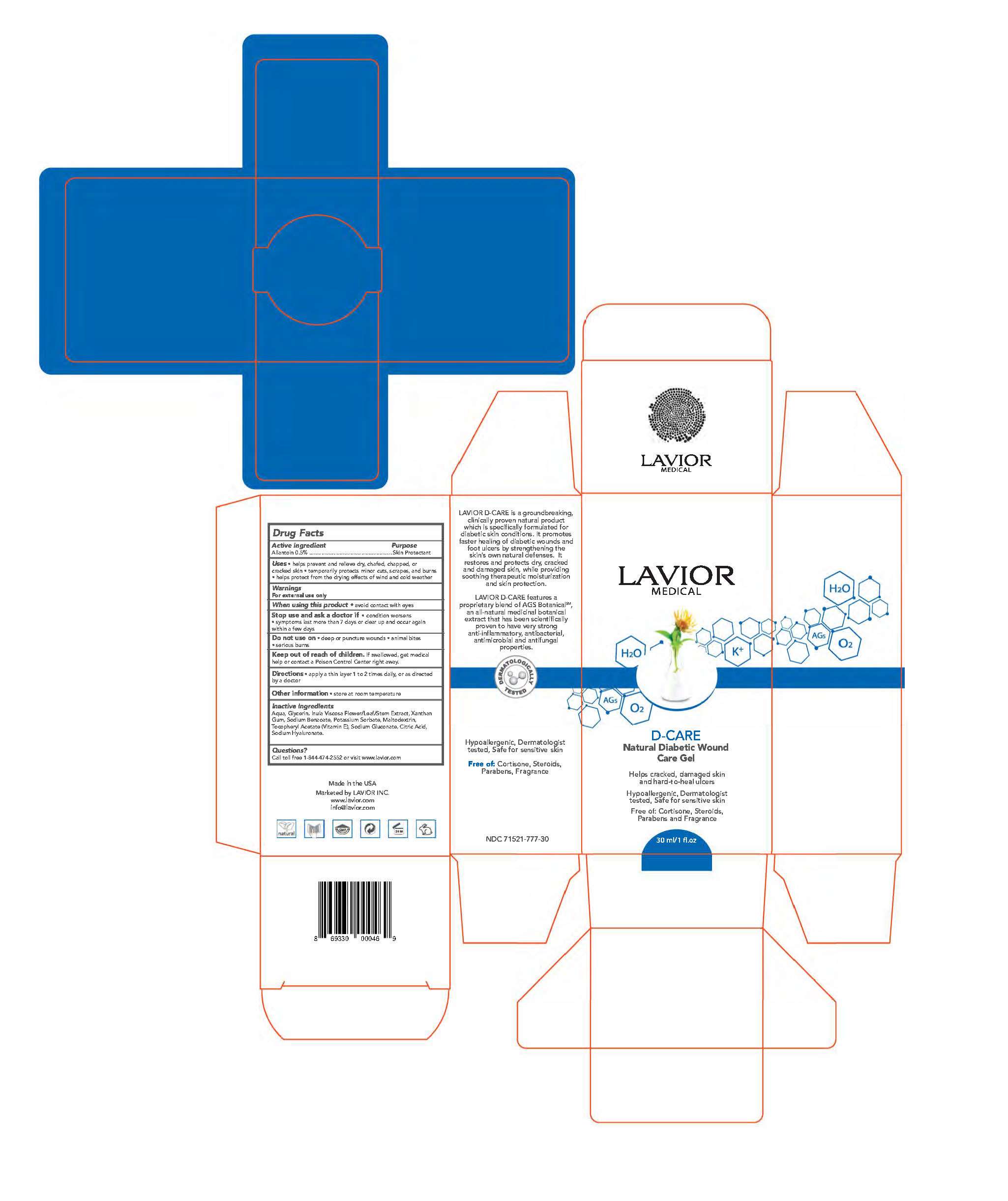
D-Care by Lavior Inc. D-CARE- allantoin gel
D-Care by
Drug Labeling and Warnings
D-Care by is a Otc medication manufactured, distributed, or labeled by Lavior Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- Uses:
- WARNINGS
- WHEN USING
- STOP USE
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
D-CARE
allantoin gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71521-777 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength SODIUM BENZOATE (UNII: OJ245FE5EU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) DITTRICHIA VISCOSA WHOLE (UNII: 3SYW69FH88) HYALURONATE SODIUM (UNII: YSE9PPT4TH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) XANTHAN GUM (UNII: TTV12P4NEE) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) MALTODEXTRIN (UNII: 7CVR7L4A2D) SODIUM GLUCONATE (UNII: R6Q3791S76) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71521-777-30 1 in 1 BOX 08/01/2017 1 30 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 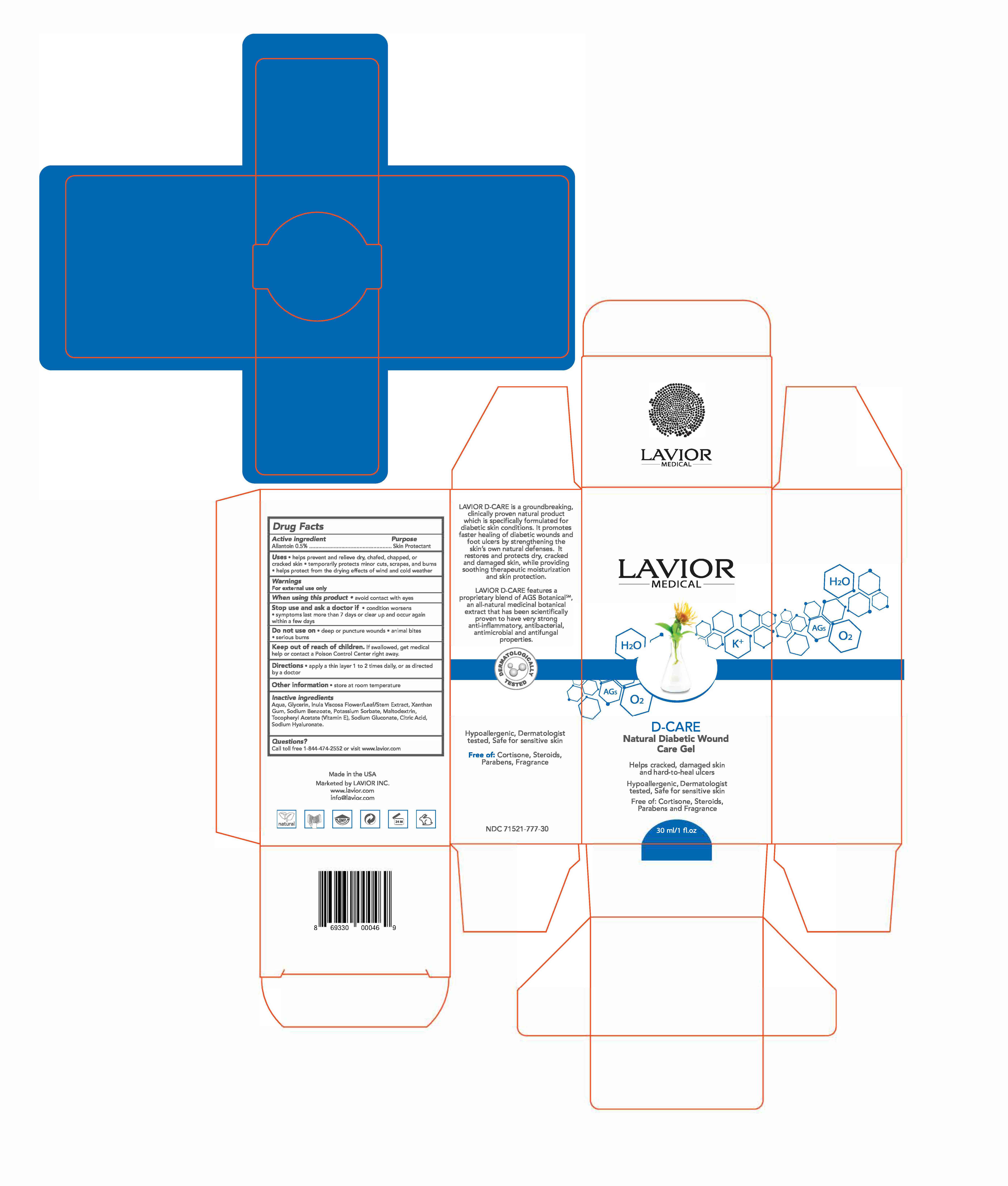
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 08/01/2017 Labeler - Lavior Inc. (080685327) Establishment Name Address ID/FEI Business Operations Lavior Inc. 080685327 manufacture(71521-777)
Trademark Results [D-Care]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 D-CARE 79324954 not registered Live/Pending |
DANAMECO MEDICAL JOINT STOCK CORPORATION 2021-01-26 |
 D-CARE 78558482 3054643 Dead/Cancelled |
CITIZENS BANK 2005-02-02 |
 D-CARE 74714733 2045000 Dead/Cancelled |
PureTek Corporation 1995-08-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.