CPDA-1- anticoagulant citrate phosphate dextrose adenine solution
CPDA-1 by
Drug Labeling and Warnings
CPDA-1 by is a Prescription medication manufactured, distributed, or labeled by Fenwal, Inc., Fenwal International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Instructions for Blood Collection Using (CPDA-1) BLOOD-PACK™ Unit
Rx Only
Integral Donor Tube (IDT)
Use aseptic technique.
Caution: Do not use unless the solutions are clear.
- 1. Identify BLOOD-PACK™ unit using appropriate donor identification system.
- 2. Adjust donor scale to desired collection weight/volume.
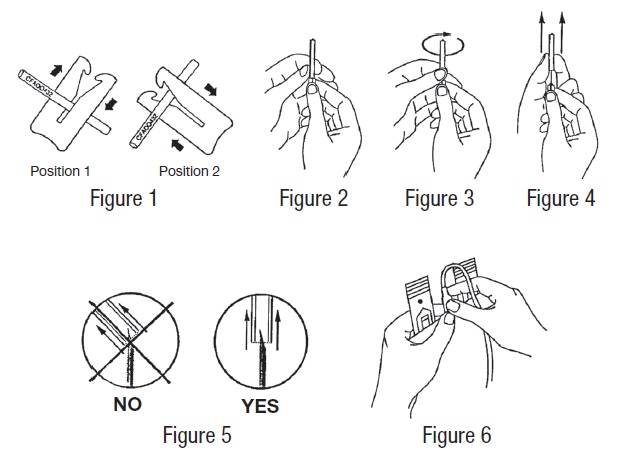
- 3. Suspend primary container from donor scale as far as possible below donor arm and clamp donor tubing at Position 1 (see Figure 1). Use one of the clamps that come inside the aluminum pouch.
- 4. Apply pressure to donor’s arm and disinfect site of venipuncture.
- 5. If blood pressure cuff is used, inflate to approximately 60 mm Hg.
- 6. Remove needle cover per instructions below:
- a) Hold the needle hub upwards. With the other hand, grasp the base of the needle cover (Figure 2), twist approximately 1/4 turn to break tamper evident seal (Figure 3).
- b) Remove needle cover (Figure 4), be careful not to drag cover across the needle point (Figure 5).
- 7. Perform venipuncture, appropriately secure donor needle and/or tubing and move clamp to Position 2 (Figure 1).
- 8. Mix blood and anticoagulant at several intervals during collection and immediately after collection.
- 9. Collect labeled volume of blood. Anticoagulant volume is sufficient for labeled volume ± 10%.
- 10. Place clamp back to Position 1 (Figure 1).
- 11. Seal donor tubing between clamp and primary container (a hand sealer clamp may be used). Dispose of needle and tubing appropriately. Cut between clamp and clip/seal; collect any needed samples using standard techniques.
- 12. Release pressure on donor’s arm and withdraw needle.
- 13. Strip blood from donor tubing into container, mix and allow the tubing to refill; repeat once. Seal at X marks on donor tubing to provide numbered aliquots of anticoagulated blood for typing or crossmatching.
- 14. Store suspended Red Blood Cells between 1 and 6°C.
- 15. Infuse suspended Red Blood Cells within 35 days of collection.
NOTE:
In order to establish fluid transfer from primary container to secondary system, hold the cannula by its base with one hand, and with the other hand break the cannula by bending it 90º in one direction, then 180º in the opposite direction (Figure 6).
For further processing, use standard component processing and storage techniques.
Dispose of containers and materials into appropriate biohazardous waste containers following established procedures.
Sterile, non-pyrogenic fluid path. Steam sterilized. Single use only.
Store at Controlled Room Temperature.
USP Definition of “Controlled Room Temperature”
United States Pharmacopeia, General Notices.
United States Pharmacopeial Convention, Inc.
12601 Twinbrook Parkway, Rockville, MD Manufacturer
Manufacturer Manufactured by:
Manufactured by:
Fenwal International, Inc.
Road 357, Km. 0.8
Maricao, PR 00606Made in USA
Imported and distributed in India by:
Fenwal India Pvt Ltd
Upper Ground Floor, Tower B
DLF Building No. 10, DLF Cyber City
DLF Phase-II, Gurgaon 122 002,
Haryana, India
Import License No.: FF-504-14890Imported and distributed in Indonesia by:
PT.Medquest Jaya Global
Menara Salemba 6th Floor
Jl.Salemba Raya Kav 5-5A
Jakarta-Indonesia 10440
Reg. No.: DEPKES RI AKL 20209902358Imported and distributed in Thailand by:
Fenwal (Thailand) Ltd.
17th Fl. Thanapoom Tower,
1550 New Petchburi Rd., Makasan
Rajthevi, Bangkok 10400
ThailandImported and distributed in Venezuela by:
SOLUCARE GT, C.A. RIF J-30512494-9
en La Urbina, Caracas,
Republica Bolivariana de Venezuela
TELF (0212) 2436663
Registrado en el MPPS bajo el No:07-19-07-227 REV: A 05/2012
FENWAL and BLOOD-PACK are trademarks of Fenwal, Inc.
© 2012 Fenwal, Inc. All rights reserved.
- PACKAGE/LABEL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CPDA-1
anticoagulant citrate phosphate dextrose adenine solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0942-6336 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dextrose Monohydrate (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) Dextrose Monohydrate 2.01 g in 63 mL Trisodium Citrate Dihydrate (UNII: B22547B95K) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 1.66 g in 63 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 206 mg in 63 mL Sodium Phosphate, Monobasic, Monohydrate (UNII: 593YOG76RN) (PHOSPHATE ION - UNII:NK08V8K8HR) Sodium Phosphate, Monobasic, Monohydrate 140 mg in 63 mL Adenine (UNII: JAC85A2161) (Adenine - UNII:JAC85A2161) Adenine 17.3 mg in 63 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0942-6336-03 63 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN770420 06/21/2010 Labeler - Fenwal, Inc. (794519020) Establishment Name Address ID/FEI Business Operations Fenwal International, Inc. 091164590 MANUFACTURE(0942-6336)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
