Eye Wash by NuCare Pharmaceuticals,Inc. / NuCare Pharnaceuticals,Inc. Eye Wash
Eye Wash by
Drug Labeling and Warnings
Eye Wash by is a Otc medication manufactured, distributed, or labeled by NuCare Pharmaceuticals,Inc., NuCare Pharnaceuticals,Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
EYE WASH- water solution
NuCare Pharmaceuticals,Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Eye Wash
Uses
washes the eye to help relieve
- irritation
- stinging
- discomfort
- itching
by removing
- loose foreign material
- air pollutants (smog or pollen)
- chlorinated water
Warnings
Do not use
- if you have open wounds in or near the eyes, Get medical help right away.
- if solution changes color or becomes cloudy
Directions
Remove contact lenses before using.
- Do not touch tip of container to any surface to avoid contamination.
- Replace cap after use.

------------------------------------------------------------------------------
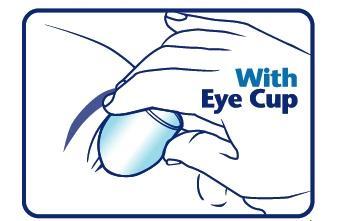
When using an eye cup
Eye wash can be used with or without the cup
- flush the affected eye as needed
- control the rate of flow of solution by pressure on the bottle
When using an eye cup
- rinse the cup with Eye Wash immediately before each use
- avoid contamination of the rim and inside surfaces of the cup
- fill the cup half full with Eye Wash Solution and apply the cup to the affected eye(s), pressing tightly to prevent spillage
- tilt the head backward. Open eyelids wide and rotate eyeball to thoroughly wash the eye
- rinse cup with clean water after each use
Inactive ingredients
boric acid, sodium borate, sodium chloride, hydrochloric acid PRESERVATIVE ADDED: edetate disodium, polyhexamethylene biguanide
| EYE WASH
water solution |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - NuCare Pharmaceuticals,Inc. (010632300) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NuCare Pharnaceuticals,Inc. | 010632300 | relabel(68071-1871) | |
