Clinol SANITIZING HAND WIPES
Clinol SANITIZING HAND WIPES by
Drug Labeling and Warnings
Clinol SANITIZING HAND WIPES by is a Otc medication manufactured, distributed, or labeled by COSMO KIMYA KOZMETIK TEKSTIL SANAYI VE TICARET LIMITED SIRKETI. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CLINOL SANITIZING HAND WIPES- benzalkonium chloride swab
COSMO KIMYA KOZMETIK TEKSTIL SANAYI VE TICARET LIMITED SIRKETI
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Clinol SANITIZING HAND WIPES
Warnings
For external use only.
Do not use if you are allergic to any of the ingredients.
When using this product do not get into eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs.
Directions
Adults and children 2 years and over:
- apply to hands.
- allow skin to dry without wiping.
Children under 2 years: ask a doctor before use.
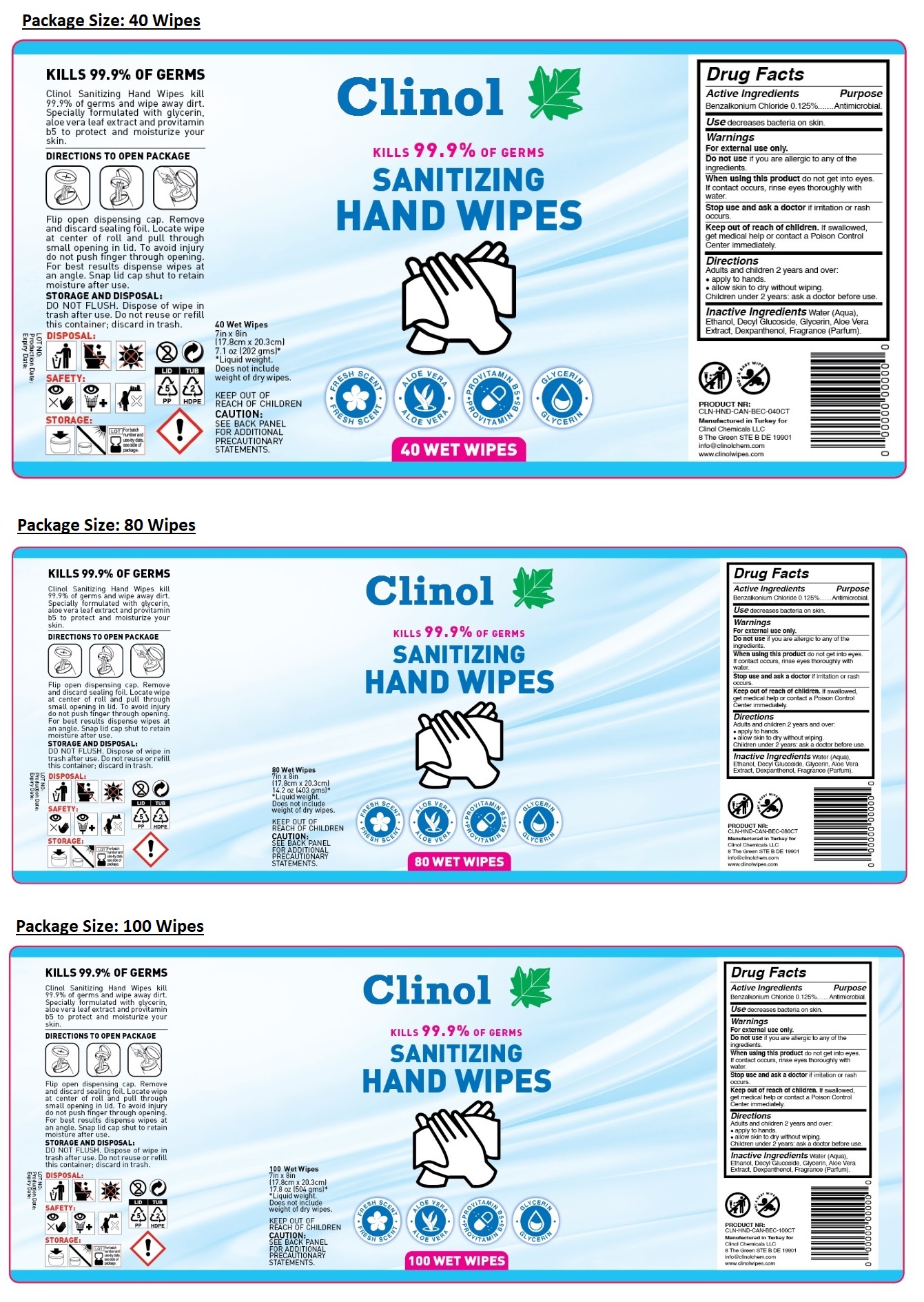
Inactive Ingredients
Water (Aqua), Ethanol, Decyl Glucoside, Glycerin, Aloe Vera Extract, Dexpanthenol, Fragrance (Parfum).
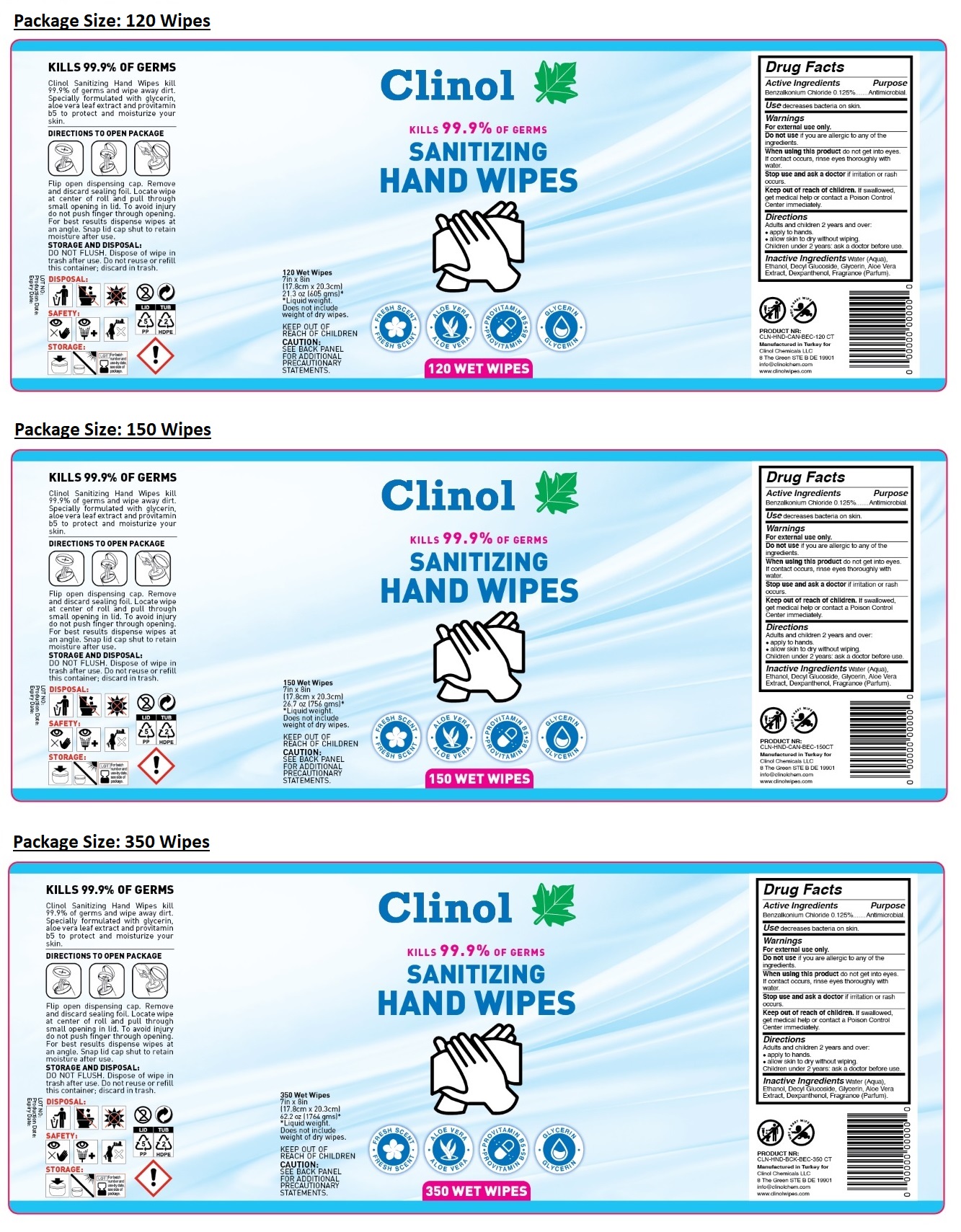
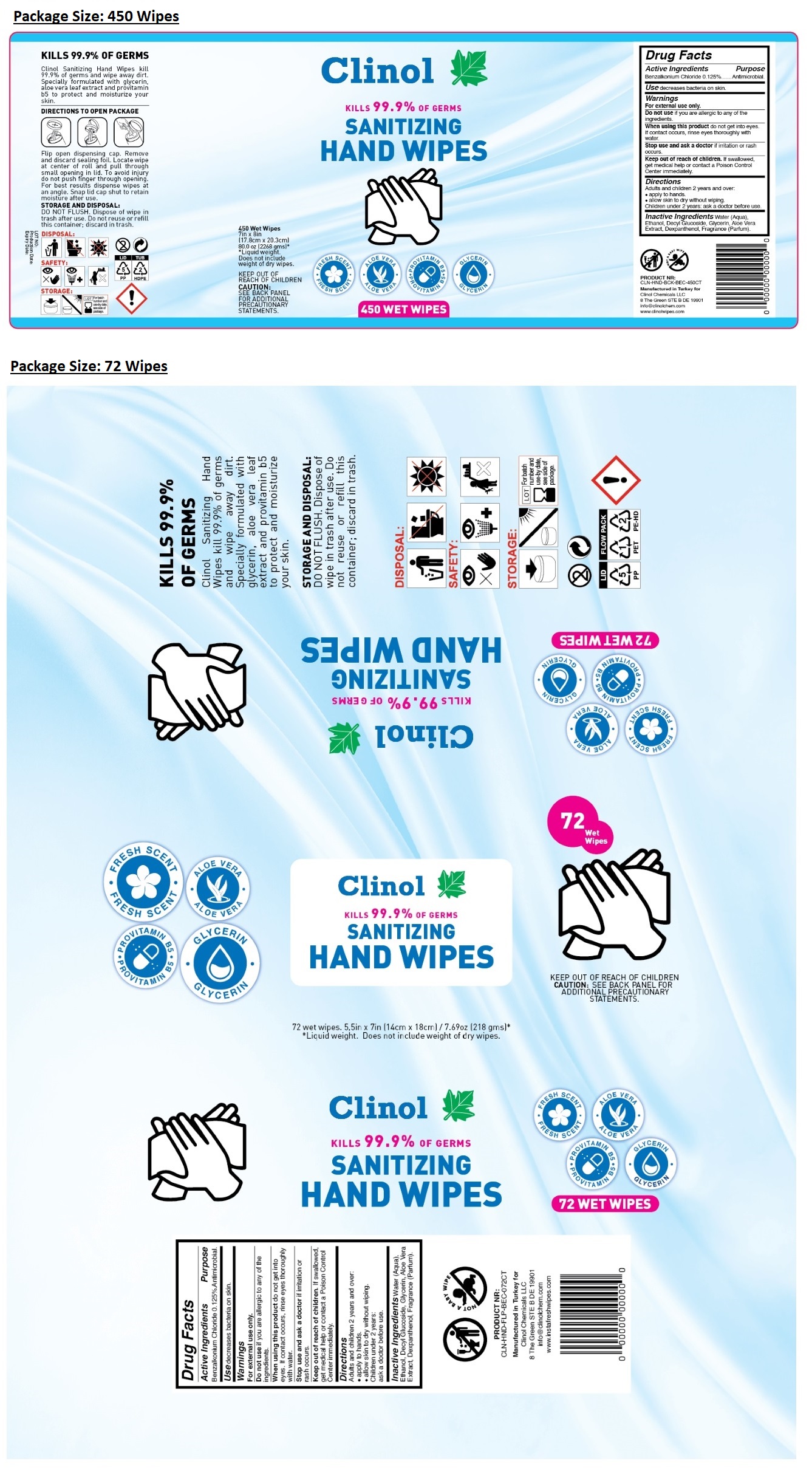
KILLS 99.9% OF GERMS
- FRESH SCENT
- ALOE VERA
- PROVITAMIN B5
- GLYCERIN
Clinol Sanitizing Hand Wipes kill 99.9% of germs and wipe away dirt. Specially formulated with glycerin, aloe vera leaf extract and provitamin b5 to protect and moisturize your skin.
DIRECTIONS TO OPEN PACKAGE
Flip open dispensing cap. Remove and discard sealing foil. Locate wipe at center of roll and pull through small opening in lid. To avoid injury do not push finger through opening. For best results dispense wipes at an angle. Snap lid cap shut to retain moisture after use.
STORAGE AND DISPOSAL: DO NOT FLUSH. Dispose of wipe in trash after use. Do not reuse or refill this container; discard in trash.
KEEP OUT OF REACH OF CHILDREN
CAUTION: SEE BACK PANEL FOR ADDITIONAL PRECAUTIONARY STATEMENTS.
NOT A BABY WIPE
Manufactured in Turkey for
Clinol Chemicals LLC
8 The Green STE B DE 19901
info@clinolchem.com
www.clinolwipes.com
| CLINOL SANITIZING HAND WIPES
benzalkonium chloride swab |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - COSMO KIMYA KOZMETIK TEKSTIL SANAYI VE TICARET LIMITED SIRKETI (354641685) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| COSMO KIMYA KOZMETIK TEKSTIL SANAYI VE TICARET LIMITED SIRKETI | 354641685 | manufacture(81388-001) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.