Montelukast by NuCare Pharmaceuticals,Inc. MONTELUKAST tablet
Montelukast by
Drug Labeling and Warnings
Montelukast by is a Prescription medication manufactured, distributed, or labeled by NuCare Pharmaceuticals,Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MONTELUKAST SODIUM TABLETS safely and effectively. See full prescribing information for MONTELUKAST SODIUM TABLETS.
MONTELUKAST SODIUM tablets, for oral use
Initial U.S. Approval: 1998RECENT MAJOR CHANGES
Warnings and Precautions, Neuropsychiatric Events ( 5.4) 12/2016
INDICATIONS AND USAGE
Montelukast sodium tablets are a leukotriene receptor antagonist indicated for:
Prophylaxis and chronic treatment of asthma in patients 15 years of age and older ( 1.1).
Acute prevention of exercise-induced bronchoconstriction (EIB) in patients 15 years of age and older ( 1.2).
Relief of symptoms of allergic rhinitis (AR): seasonal allergic rhinitis (SAR) in patients 15 years of age and older, and perennial allergic rhinitis (PAR) in patients 15 years of age and older ( 1.3).DOSAGE AND ADMINISTRATION
Administration (by indications):
- Asthma ( 2.1): Once daily in the evening for patients 15 years of age and older.
- Acute prevention of EIB ( 2.2): 10 mg tablet at least 2 hours before exercise for patients 15 years of age and older.
- Seasonal allergic rhinitis ( 2.3): Once daily for patients 15 years and older.
- Perennial allergic rhinitis ( 2.3): Once daily for patients 15 years and older.
Dosage (by age):
- 15 years and older: one 10-mg tablet.
Patients with both asthma and allergic rhinitis should take only one dose daily in the evening ( 2.4).
DOSAGE FORMS AND STRENGTHS
Montelukast sodium Film-Coated Tablets, 10 mg (3)
CONTRAINDICATIONS
Hypersensitivity to any component of this product ( 4).
WARNINGS AND PRECAUTIONS
- Do not prescribe montelukast sodium to treat an acute asthma attack.
- Advise patients to have appropriate rescue medication available ( 5.1).
- Inhaled corticosteroid may be reduced gradually. Do not abruptly substitute montelukast sodium for inhaled or oral corticosteroids ( 5.2).
- Patients with known aspirin sensitivity should continue to avoid aspirin or non-steroidal anti-inflammatory agents while taking montelukast sodium( 5.3).
- Neuropsychiatric events have been reported with montelukast sodium. Instruct patients to be alert for neuropsychiatric events. Evaluate the risks and benefits of continuing treatment with montelukast sodium if such events occur ( 5.4 and 6.2).
- Systemic eosinophilia, sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, has been reported. These events have been sometimes associated with the reduction of oral corticosteroid therapy ( 5.5 and 6.2).
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 5% and greater than placebo listed in descending order of frequency): upper respiratory infection, fever, headache, pharyngitis, cough, abdominal pain, diarrhea, otitis media, influenza, rhinorrhea, sinusitis, otitis ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Macleods Pharma USA, Inc., at 1-888-943-3210 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS & USAGE
1.1 Asthma
1.2 Exercise-Induced Bronchoconstriction (EIB)
1.3 Allergic Rhinitis
2 DOSAGE & ADMINISTRATION
2.1 Asthma
2.2 Exercise-Induced Bronchoconstriction (EIB)
2.3 Allergic Rhinitis
2.4 Asthma and Allergic Rhinitis
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Acute Asthma
5.2 Concomitant Corticosteroid Use
5.3 Aspirin Sensitivity
5.4 Neuropsychiatric Events
5.5 Eosinophilic Conditions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Insufficiency
8.7 Renal Insufficiency
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Asthma
14.2 Exercise-Induced Bronchoconstriction (EIB)
14.3 Allergic Rhinitis (Seasonal and Perennial)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS & USAGE
1.1 Asthma
Montelukast sodium tablets are indicated for the prophylaxis and chronic treatment of asthma in patients 15 years of age and older.
-
2 DOSAGE & ADMINISTRATION
2.1 Asthma
Montelukast sodium should be taken once daily in the evening. The following dose is are recommended:
For adults and adolescents 15 years of age and older: one 10-mg tablet.
Safety and effectiveness in pediatric patients less than 12 months of age with asthma have not been established.
There have been no clinical trials in patients with asthma to evaluate the relative efficacy of morning versus evening dosing. The pharmacokinetics of montelukast are similar whether dosed in the morning or evening. Efficacy has been demonstrated for asthma when montelukast was administered in the evening without regard to time of food ingestion.2.2 Exercise-Induced Bronchoconstriction (EIB)
For prevention of EIB, a single 10 mg dose of montelukast should be taken at least 2 hours before exercise.
The following dose is recommended :
For adults and adolescents 15 years of age and older: one 10-mg tablet.An additional dose of montelukast should not be taken within 24 hours of a previous dose. Patients already taking montelukast sodium daily for another indication (including chronic asthma) should not take an additional dose to prevent EIB. All patients should have available for rescue a short-acting ß-agonist. Safety and effectiveness in patients younger than 15 years of age have not been established. Daily administration of montelukast sodium for the chronic treatment of asthma has not been established to prevent acute episodes of EIB.
2.3 Allergic Rhinitis
For allergic rhinitis, montelukast sodium should be taken once daily. Efficacy was demonstrated for seasonal allergic rhinitis when montelukast was administered in the morning or the evening without regard to time of food ingestion. The time of administration may be individualized to suit patient needs.
The following dose for the treatment of symptoms of seasonal allergic rhinitis are recommended:
For adults and adolescents 15 years of age and older: one 10-mg tablet.
Safety and effectiveness in pediatric patients younger than 2 years of age with seasonal allergic rhinitis have not been established.
The following dose for the treatment of symptoms of perennial allergic rhinitis are recommended:
For adults and adolescents 15 years of age and older: one 10-mg tablet.
Safety and effectiveness in pediatric patients younger than 6 months of age with perennial allergic rhinitis have not been established.
- 3 DOSAGE FORMS & STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Acute Asthma
Montelukast sodium is not indicated for use in the reversal of bronchospasm in acute asthma attacks, including status asthmaticus. Patients should be advised to have appropriate rescue medication available. Therapy with montelukast sodium can be continued during acute exacerbations of asthma. Patients who have exacerbations of asthma after exercise should have available for rescue a short-acting inhaled ß-agonist.
5.2 Concomitant Corticosteroid Use
While the dose of inhaled corticosteroid may be reduced gradually under medical supervision, montelukast sodium should not be abruptly substituted for inhaled or oral corticosteroids.
5.3 Aspirin Sensitivity
Patients with known aspirin sensitivity should continue avoidance of aspirin or non-steroidal anti-inflammatory agents while taking montelukast sodium. Although montelukast sodium is effective in improving airway function in asthmatics with documented aspirin sensitivity, it has not been shown to truncate bronchoconstrictor response to aspirin and other non-steroidal anti-inflammatory drugs in aspirin-sensitive asthmatic patients [see Clinical Studies (14.1)].
5.4 Neuropsychiatric Events
Patients and prescribers should be alert for neuropsychiatric events. Patients should be instructed to notify their prescriber if these changes occur. Prescribers should carefully evaluate the risks and benefits of continuing treatment with montelukast sodium if such events occur . Neuropsychiatric events have been reported in adult, adolescent, and pediatric patients taking montelukast sodium. Post-marketing reports with montelukast use include agitation, aggressive behavior or hostility, anxiousness, depression, disorientation, disturbance in attention, dream abnormalities, hallucinations, insomnia, irritability, memory impairment, restlessness, somnambulism, suicidal thinking and behavior (including suicide), tic, and tremor. The clinical details of some post-marketing reports involving montelukast sodium appear consistent with a drug-induced effect.
Patients and prescribers should be alert for neuropsychiatric events. Patients should be instructed to notify their prescriber if these changes occur. Prescribers should carefully evaluate the risks and benefits of continuing treatment with montelukast sodium if such events occur [see Adverse Reactions (6.2)] .5.5 Eosinophilic Conditions
Patients with asthma on therapy with montelukast sodium may present with systemic eosinophilia, sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These events have been sometimes associated with the reduction of oral corticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal association between montelukast sodium and these underlying conditions has not been established [see Adverse Reactions ( 6.2)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. In the following description of clinical trials experience, adverse reactions are listed regardless of causality assessment.
The most common adverse reactions (incidence ≥5% and greater than placebo; listed in descending order of frequency) in controlled clinical trials were: upper respiratory infection, fever, headache, pharyngitis, cough, abdominal pain, diarrhea, otitis media, influenza, rhinorrhea, sinusitis, otitis.
Adults and Adolescents 15 Years of Age and Older with Asthma
Montelukast sodium has been evaluated for safety in approximately 2950 adult and adolescent patients 15 years of age and older in clinical trials. In placebo-controlled clinical trials, the following adverse experiences reported with montelukast occurred in greater than or equal to 1% of patients and at an incidence greater than that in patients treated with placebo:
TABLE 1: Adverse Experiences Occurring in ≥1% of Patients with an Incidence Greater than that in Patients Treated with Placebo
- * Number of patients tested (montelukast sodium and placebo, respectively): ALT and AST, 1935, 1170; pyuria, 1924, 1159.
Montelukast 10 mg/day
(%)
(n=1955)
Placebo
(%)
(n=1180)
Body As A Whole Pain, abdominal 2.9
2.5 Asthenia/fatigue 1.8 1.2 Fever 1.5 0.9 Trauma 1.0 0.8 Digestive System Disorders Dyspepsia 2.1
1.1 Pain, dental 1.7 1.0 Gastroenteritis, infectious 1.5 0.5 Nervous System/Psychiatric Headache 18.4
18.1 Dizziness 1.9 1.4 Respiratory System Disorders Influenza 4.2
3.9 Cough 2.7 2.4 Congestion, nasal 1.6 1.3 Skin/Skin Appendages Disorder Rash 1.6
1.2 Laboratory Adverse Experiences * ALT increased 2.1
2.0 AST increased 1.6 1.2 Pyuria 1.0 0.9
The frequency of less common adverse events was comparable between montelukast sodium and placebo.
The safety profile of montelukast sodium, when administered as a single dose for prevention of EIB in adult and adolescent patients 15 years of age and older, was consistent with the safety profile previously described for montelukast sodium.
Cumulatively, 569 patients were treated with montelukast sodium for at least 6 months, 480 for one year, and 49 for two years in clinical trials. With prolonged treatment, the adverse experience profile did not significantly change.
Adults and Adolescents 15 Years of Age and Older with Seasonal Allergic Rhinitis
Montelukast sodium has been evaluated for safety in 2199 adult and adolescent patients 15 years of age and older in clinical trials. Montelukast sodium administered once daily in the morning or in the evening had a safety profile similar to that of placebo. In placebo-controlled clinical trials, the following event was reported with montelukast sodium with a frequency ≥ 1% and at an incidence greater than placebo: upper respiratory infection, 1.9% of patients receiving montelukast sodium vs. 1.5% of patients receiving placebo. In a 4-week, placebo-controlled clinical study, the safety profile was consistent with that observed in 2-week studies. The incidence of somnolence was similar to that of placebo in all studies.
Adults and Adolescents 15 Years of Age and Older with Perennial Allergic Rhinitis
Montelukast sodium has been evaluated for safety in 3357 adult and adolescent patients 15 years of age and older with perennial allergic rhinitis of whom 1632 received montelukast sodium in two, 6-week, clinical studies. Montelukast sodium administered once daily had a safety profile consistent with that observed in patients with seasonal allergic rhinitis and similar to that of placebo. In these two studies, the following events were reported with montelukast sodium with a frequency ≥ 1% and at an incidence greater than placebo: sinusitis, upper respiratory infection, sinus headache, cough, epistaxis, and increased ALT. The incidence of somnolence was similar to that of placebo.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of montelukast sodium. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: increased bleeding tendency, thrombocytopenia.
Immune system disorders: hypersensitivity reactions including anaphylaxis, hepatic eosinophilic infiltration.
Psychiatric disorders: agitation including aggressive behavior or hostility, anxiousness, depression, disorientation, disturbance in attention, dream abnormalities, hallucinations, insomnia, irritability, restlessness, somnambulism, suicidal thinking and behavior (including suicide), tic, and tremor [ see Warnings and Precautions (5.4) ].
Nervous system disorders: drowsiness, paraesthesia/hypoesthesia, seizures.
Cardiac disorders: palpitations.
Respiratory, thoracic and mediastinal disorders: epistaxis, pulmonary eosinophilia.
Gastrointestinal disorders: diarrhea, dyspepsia, nausea, pancreatitis, vomiting.
Hepatobiliary disorders: Cases of cholestatic hepatitis, hepatocellular liver-injury, and mixed-pattern liver injury have been reported in patients treated with montelukast sodium. Most of these occurred in combination with other confounding factors, such as use of other medications, or when montelukast sodium was administered to patients who had underlying potential for liver disease such as alcohol use or other forms of hepatitis.
Skin and subcutaneous tissue disorders: angioedema, bruising, erythema multiforme, erythema nodosum,pruritus, Stevens-Johnson syndrome/toxic epidermal necrolysis, urticaria.
Musculoskeletal and connective tissue disorders: arthralgia, myalgia including muscle cramps.
Renal and urinary disorders: enuresis in children.
General disorders and administration site conditions: edema.
Patients with asthma on therapy with montelukast sodium may present with systemic eosinophilia, sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These events have been sometimes associated with the reduction of oral corticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients [ see Warnings and Precautions (5.5) ].
-
7 DRUG INTERACTIONS
No dose adjustment is needed when montelukast sodium is co-administered with theophylline, prednisone, prednisolone, oral contraceptives, terfenadine, digoxin,
warfarin, gemfibrozil, itraconazole, thyroid hormones, sedative hypnotics, non-steroidal anti-inflammatory agents, benzodiazepines, decongestants, and Cytochrome P450 (CYP) enzyme inducers [see Clinical Pharmacology (12.3) ].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B: There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, montelukast sodium should be used during pregnancy only if clearly needed.
Teratogenic Effect: No teratogenicity was observed in rats and rabbits at doses approximately 100 and 110 times, respectively, the maximum recommended daily oral dose in adults based on AUCs [see Nonclinical Toxicology (13.2) ].
During worldwide marketing experience, congenital limb defects have been rarely reported in the offspring of women being treated with montelukast sodium during pregnancy. Most of these women were also taking other asthma medications during their pregnancy. A causal relationship between these events and montelukast sodium has not been established.
8.3 Nursing Mothers
Studies in rats have shown that montelukast is excreted in milk. It is not known if montelukast is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when montelukast sodium is given to a nursing mother.
8.4 Pediatric Use
The safety and effectiveness in pediatric patients below the age of 12 months with asthma and 6 months with perennial allergic rhinitis have not been established. The safety and effectiveness in pediatric patients below the age of 6 years with exercise-induced bronchoconstriction have not been established.
8.5 Geriatric Use
Of the total number of subjects in clinical studies of montelukast, 3.5% were 65 years of age and over, and 0.4% were 75 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. The pharmacokinetic profile and the oral bioavailability of a single 10-mg oral dose of montelukast are similar in elderly and younger adults. The plasma half-life of montelukast is slightly longer in the elderly. No dosage adjustment in the elderly is required.
8.6 Hepatic Insufficiency
No dosage adjustment is required in patients with mild-to-moderate hepatic insufficiency [see Clinical Pharmacology (12.3)].
8.7 Renal Insufficiency
No dosage adjustment is recommended in patients with renal insufficiency [see Clinical Pharmacology (12.3) ].
-
10 OVERDOSAGE
No specific information is available on the treatment of overdosage with montelukast sodium. In chronic asthma studies, montelukast has been administered at doses up to 200 mg/day to adult patients for 22 weeks and, in short-term studies, up to 900 mg/day to patients for approximately a week without clinically important adverse experiences. In the event of overdose, it is reasonable to employ the usual supportive measures; e.g., remove unabsorbed material from the gastrointestinal tract, employ clinical monitoring, and institute supportive therapy, if required.
There have been reports of acute overdosage in post-marketing experience and clinical studies with montelukast sodium. These include reports in adults and children with a dose as high as 1000 mg. The clinical and laboratory findings observed were consistent with the safety profile in adults and pediatric patients. There were no adverse experiences in the majority of overdosage reports. The most frequently occurring adverse experiences were consistent with the safety profile of montelukast sodium and included abdominal pain, somnolence, thirst, headache, vomiting and psychomotor hyperactivity.
It is not known whether montelukast is removed by peritoneal dialysis or hemodialysis. -
11 DESCRIPTION
Montelukast sodium, USP the active ingredient in montelukast sodium tablets, is a selective and orally active leukotriene receptor antagonist that inhibits the cysteinyl leukotriene CysLT 1 receptor.
Montelukast sodium, USP is described chemically as [R-(E)]-1-[[[1-[3-[2-(7-chloro-2-quinolinyl)ethenyl] phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl] propyl]thio]methyl]cyclopropaneacetic acid, monosodium salt.
The molecular formula is C 35H 35ClNNaO 3S, and its molecular weight is 608.18. The structural formula is:
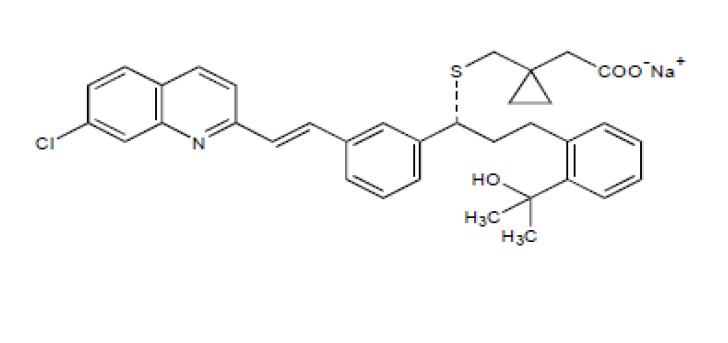
Montelukast sodium, USP is a hygroscopic, optically active, white to off-white powder. Montelukast sodium is freely soluble in ethanol, methanol, and water and practically insoluble in acetonitrile.
Each 10-mg film-coated montelukast tablet contains 10.4 mg montelukast sodium, USP which is equivalent to 10 mg of montelukast, and the following inactive ingredients: microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, hydroxypropyl cellulose, disodium edetate and magnesium stearate. The film coating consists of: hydroxypropyl methylcellulose, hydroxypropyl cellulose, titanium dioxide, red ferric oxide and yellow ferric oxide. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The cysteinyl leukotrienes (LTC 4, LTD 4, LTE 4) are products of arachidonic acid metabolism and are released from various cells, including mast cells and eosinophils. These eicosanoids bind to cysteinyl leukotriene (CysLT) receptors. The CysLT type-1 (CysLT 1) receptor is found in the human airway (including airway smooth muscle cells and airway macrophages) and on other pro-inflammatory cells (including eosinophils and certain myeloid stem cells). CysLTs have been correlated with the pathophysiology of asthma and allergic rhinitis. In asthma, leukotriene-mediated effects include airway edema, smooth muscle contraction, and altered cellular activity associated with the inflammatory process. In allergic rhinitis, CysLTs are released from the nasal mucosa after allergen exposure during both early- and late-phase reactions and are associated with symptoms of allergic rhinitis.
Montelukast is an orally active compound that binds with high affinity and selectivity to the CysLT 1 receptor (in preference to other pharmacologically important airway receptors, such as the prostanoid, cholinergic, or β-adrenergic receptor). Montelukast inhibits physiologic actions of LTD 4 at the CysLT 1 receptor without any agonist activity.12.2 Pharmacodynamics
Montelukast causes inhibition of airway cysteinyl leukotriene receptors as demonstrated by the ability to inhibit bronchoconstriction due to inhaled LTD 4 in asthmatics. Doses as low as 5 mg cause substantial blockage of LTD 4-induced bronchoconstriction. In a placebo-controlled, crossover study (n=12), montelukast sodium inhibited early- and late-phase bronchoconstriction due to antigen challenge by 75% and 57%, respectively.
The effect of montelukast sodium on eosinophils in the peripheral blood was examined in clinical trials. In patients with asthma aged 2 years and older who received montelukast, a decrease in mean peripheral blood eosinophil counts ranging from 9% to 15% was noted, compared with placebo, over the double-blind treatment periods. In patients with seasonal allergic rhinitis aged 15 years and older who received montelukast sodium, a mean increase of 0.2% in peripheral blood eosinophil counts was noted, compared with a mean increase of 12.5% in placebo-treated patients, over the double-blind treatment periods; this reflects a mean difference of 12.3% in favor of montelukast sodium. The relationship between these observations and the clinical benefits of montelukast noted in the clinical trials is not known [see Clinical Studies (14)].12.3 Pharmacokinetics
Absorption
Montelukast is rapidly absorbed following oral administration. After administration of the 10-mg film-coated tablet to fasted adults, the mean peak montelukast plasma concentration (C max) is achieved in 3 to 4 hours (T max). The mean oral bioavailability is 64%. The oral bioavailability and C max are not influenced by a standard meal in the morning.
The safety and efficacy of montelukast in patients with asthma were demonstrated in clinical trials in which the 10-mg film-coated tablet formulation was administered in the evening without regard to the time of food ingestion. The safety and efficacy of montelukast in patients with seasonal allergic rhinitis were demonstrated in clinical trials in which the 10-mg film-coated tablet was administered in the morning or evening without regard to the time of food ingestion.
The comparative pharmacokinetics of montelukast when administered as two 5-mg chewable tablets versus one 10-mg film-coated tablet have not been evaluated.
Distribution
Montelukast is more than 99% bound to plasma proteins. The steady state volume of distribution of montelukast averages 8 to 11 liters. Studies in rats with radiolabeled montelukast indicate minimal distribution across the blood-brain barrier. In addition, concentrations of radiolabeled material at 24 hours postdose were minimal in all other tissues.
Metabolism
Montelukast is extensively metabolized. In studies with therapeutic doses, plasma concentrations of metabolites of montelukast are undetectable at steady state in adults and pediatric patients.In vitro studies using human liver microsomes indicate that CYP3A4, 2C8, and 2C9 are involved in the metabolism of montelukast. At clinically relevant concentrations, 2C8 appears to play a major role in the metabolism of montelukast.
Elimination
The plasma clearance of montelukast averages 45 mL/min in healthy adults. Following an oral dose of radiolabeled montelukast, 86% of the radioactivity was recovered in 5-day fecal collections and <0.2% was recovered in urine. Coupled with estimates of montelukast oral bioavailability, this indicates that montelukast and its metabolites are excreted almost exclusively via the bile.
In several studies, the mean plasma half-life of montelukast ranged from 2.7 to 5.5 hours in healthy young adults. The pharmacokinetics of montelukast are nearly linear for oral doses up to 50 mg. During once-daily dosing with 10-mg montelukast, there is little accumulation of the parent drug in plasma (14%).
Special Populations
Hepatic Insufficiency: Patients with mild-to-moderate hepatic insufficiency and clinical evidence of cirrhosis had evidence of decreased metabolism of montelukast resulting in 41% (90% CI=7%, 85%) higher mean montelukast AUC following a single 10-mg dose. The elimination of montelukast was slightly prolonged compared with that in healthy subjects (mean half-life, 7.4 hours).
No dosage adjustment is required in patients with mild-to-moderate hepatic insufficiency. The pharmacokinetics of montelukast in patients with more severe hepatic impairment or with hepatitis have not been evaluated.
Renal Insufficiency: Since montelukast and its metabolites are not excreted in the urine, the pharmacokinetics of montelukast were not evaluated in patients with renal insufficiency. No dosage adjustment is recommended in these patients.
Gender: The pharmacokinetics of montelukast are similar in males and females.
Race: Pharmacokinetic differences due to race have not been studied.
Adolescents and Pediatric Patients: Pharmacokinetic studies evaluated the systemic exposure of the 10-mg film-coated tablets in young adults and adolescents ≥ 15 years of age.
The plasma concentration profile of montelukast following administration of the 10-mg film-coated tablet is similar in adolescents ≥ 15 years of age and young adults. The 10-mg film-coated tablet is recommended for use in patients ≥ 15 years of age.
Drug-Drug Interactions
Theophylline, Prednisone, and Prednisolone: Montelukast sodium has been administered with other therapies routinely used in the prophylaxis and chronic treatment of asthma with no apparent increase in adverse reactions. In drug-interaction studies, the recommended clinical dose of montelukast did not have clinically important effects on the pharmacokinetics of the following drugs: theophylline, prednisone, and prednisolone.
Montelukast at a dose of 10 mg once daily dosed to pharmacokinetic steady state, did not cause clinically significant changes in the kinetics of a single intravenous dose of theophylline [predominantly a cytochrome P450 (CYP) 1A2 substrate]. Montelukast at doses of ≥ 100 mg daily dosed to pharmacokinetic steady state, did not cause any clinically significant change in plasma profiles of prednisone or prednisolone following administration of either oral prednisone or intravenous prednisolone.
Oral Contraceptives, Terfenadine, Digoxin, and Warfarin: In drug interaction studies, the recommended clinical dose of montelukast did not have clinically important effects on the pharmacokinetics of the following drugs: oral contraceptives (norethindrone 1 mg/ethinyl estradiol 35 mcg), terfenadine, digoxin, and warfarin. Montelukast at doses of ≥ 100 mg daily dosed to pharmacokinetic steady state did not significantly alter the plasma concentrations of either component of an oral contraceptive containing norethindrone 1 mg/ ethinyl estradiol 35 mcg. Montelukast at a dose of 10 mg once daily dosed to pharmacokinetic steady state did not change the plasma concentration profile of terfenadine (a substrate of CYP3A4) or fexofenadine, the carboxylated metabolite, and did not prolong the QTc interval following co-administration with terfenadine 60 mg twice daily; did not change the pharmacokinetic profile or urinary excretion of immunoreactive digoxin; did not change the pharmacokinetic profile of warfarin (primarily a substrate of CYP2C9, 3A4 and 1A2) or influence the effect of a single 30-mg oral dose of warfarin on prothrombin time or the International Normalized Ratio (INR).
Thyroid Hormones, Sedative Hypnotics, Non-Steroidal Anti-Inflammatory Agents, Benzodiazepines, and Decongestants: Although additional specific interaction studies were not performed, montelukast was used concomitantly with a wide range of commonly prescribed drugs in clinical studies without evidence of clinical adverse interactions. These medications included thyroid hormones, sedative hypnotics, non-steroidal anti-inflammatory agents, benzodiazepines, and decongestants.
Cytochrome P450 (CYP) Enzyme Inducers: Phenobarbital, which induces hepatic metabolism, decreased the area under the plasma concentration curve (AUC) of montelukast approximately 40% following a single 10-mg dose of montelukast. No dosage adjustment for montelukast sodium is recommended. It is reasonable to employ appropriate clinical monitoring when potent CYP enzyme inducers, such as phenobarbital or rifampin, are co-administered with montelukast sodium.
Effect of Montelukast on Cytochrome P450 (CYP) Enzymes: Montelukast is a potent inhibitor of CYP2C8 in vitro. However, data from a clinical drug-drug interaction study involving montelukast and rosiglitazone (a probe substrate representative of drugs primarily metabolized by CYP2C8) in 12 healthy individuals demonstrated that the pharmacokinetics of rosiglitazone are not altered when the drugs are coadministered, indicating that montelukast does not inhibit CYP2C8 in vivo. Therefore, montelukast is not anticipated to alter the metabolism of drugs metabolized by this enzyme (e.g., paclitaxel, rosiglitazone, and repaglinide). Based on further in vitro results in human liver microsomes, therapeutic plasma concentrations of montelukast do not inhibit CYP 3A4, 2C9, 1A2, 2A6, 2C19, or 2D6.Cytochrome P450 (CYP) Enzyme Inhibitors: In vitro studies have shown that montelukast is a substrate of CYP 2C8, 2C9, and 3A4. Co-administration of montelukast with itraconazole, a strong CYP 3A4 inhibitor, resulted in no significant increase in the systemic exposure of montelukast. Data from a clinical drug-drug interaction study involving montelukast and gemfibrozil (an inhibitor of both CYP 2C8 and 2C9) demonstrated that gemfibrozil, at a therapeutic dose, increased the systemic exposure of montelukast by 4.4-fold. Co-administration of itraconazole, gemfibrozil, and montelukast did not further increase the systemic exposure of montelukast. Based on available clinical experience, no dosage adjustment of montelukast is required upon co-administration with gemfibrozil [ see Overdosage (10)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
No evidence of tumorigenicity was seen in carcinogenicity studies of either 2 years in Sprague-Dawley rats or 92 weeks in mice at oral gavage doses up to 200 mg/kg/day or 100 mg/kg/day, respectively. The estimated exposure in rats was approximately 120 and 75 times the AUC for adults and children, respectively, at the maximum recommended daily oral dose. The estimated exposure in mice was approximately 45 and 25 times the AUC for adults and children, respectively, at the maximum recommended daily oral dose.
Montelukast demonstrated no evidence of mutagenic or clastogenic activity in the following assays: the microbial mutagenesis assay, the V-79 mammalian cell mutagenesis assay, the alkaline elution assay in rat hepatocytes, the chromosomal aberration assay in Chinese hamster ovary cells, and in the in vivo mouse bone marrow chromosomal aberration assay.
In fertility studies in female rats, montelukast produced reductions in fertility and fecundity indices at an oral dose of 200 mg/kg (estimated exposure was approximately 70 times the AUC for adults at the maximum recommended daily oral dose). No effects on female fertility or fecundity were observed at an oral dose of 100 mg/kg (estimated exposure was approximately 20 times the AUC for adults at the maximum recommended daily oral dose). Montelukast had no effects on fertility in male rats at oral doses up to 800 mg/kg (estimated exposure was approximately 160 times the AUC for adults at the maximum recommended daily oral dose).13.2 Animal Toxicology and/or Pharmacology
Reproductive Toxicology Studies
No teratogenicity was observed at oral doses up to 400 mg/kg/day and 300 mg/kg/day in rats and rabbits, respectively. These doses were approximately 100 and 110 times the maximum recommended daily oral dose in adults, respectively, based on AUCs. Montelukast crosses the placenta following oral dosing in rats and rabbits [see Pregnancy (8.1) ]. -
14 CLINICAL STUDIES
14.1 Asthma
Adults and Adolescents 15 Years of Age and Older with Asthma
Clinical trials in adults and adolescents 15 years of age and older demonstrated there is no additional clinical benefit to montelukast doses above 10 mg once daily.
The efficacy of montelukast sodium for the chronic treatment of asthma in adults and adolescents 15 years of age and older was demonstrated in two (U.S. and Multinational) similarly designed, randomized, 12-week, double-blind, placebo-controlled trials in 1576 patients (795 treated with montelukast sodium, 530 treated with placebo, and 251 treated with active control). The median age was 33 years (range 15 to 85); 56.8% were females and 43.2% were males. The ethnic/racial distribution in these studies was 71.6% Caucasian, 17.7% Hispanic, 7.2% other origins and 3.5% Black. Patients had mild or moderate asthma and were non-smokers who required approximately 5 puffs of inhaled β-agonist per day on an “as-needed” basis. The patients had a mean baseline percent of predicted forced expiratory volume in 1 second (FEV 1) of 66% (approximate range, 40 to 90%). The co-primary endpoints in these trials were FEV 1 and daytime asthma symptoms. In both studies after 12 weeks, a random subset of patients receiving montelukast sodium was switched to placebo for an additional 3 weeks of double-blind treatment to evaluate for possible rebound effects.
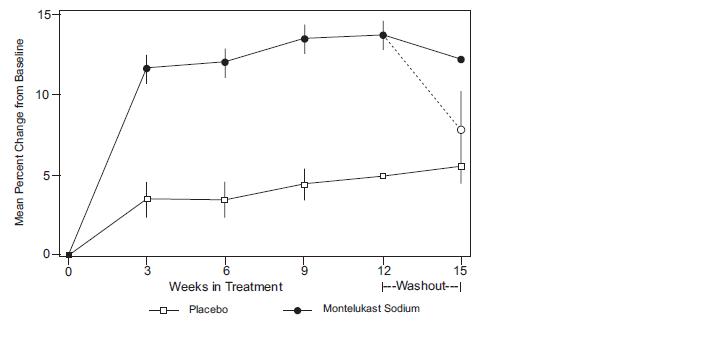
The results of the U.S. trial on the primary endpoint, morning FEV 1, expressed as mean percent change from baseline averaged over the 12-week treatment period, are shown in FIGURE 2. Compared with placebo, treatment with one montelukast sodium 10-mg tablet daily in the evening resulted in a statistically significant increase in FEV 1 percent change from baseline (13.0%-change in the group treated with montelukast sodium vs. 4.2%-change in the placebo group, p<0.001); the change from baseline in FEV 1 for montelukast sodium was 0.32 liters compared with 0.10 liters for placebo, corresponding to a between-group difference of 0.22 liters (p<0.001, 95% CI 0.17 liters, 0.27 liters). The results of the Multinational trial on FEV 1 were similar.
FIGURE 2: FEV 1 Mean Percent Change from Baseline (U.S. Trial: Montelukast Sodium N=406; Placebo N=270) (ANOVA Model)
The effect of montelukast sodium on other primary and secondary endpoints, represented by the Multinational study is shown in TABLE 2.
Results on these endpoints were similar in the US study.
TABLE 2: Effect of Montelukast sodium on Primary and Secondary Endpoints in a Multinational Placebo-controlled Trial (ANOVA Model)
- * p<0.001, compared with placebo
Endpoint
Montelukast Sodium
Placebo
N
Baseline
Mean Change from Baseline
N
Baseline
Mean Change from Baseline
Daytime Asthma Symptoms (0 to 6 scale)
372
2.35
-0.49 *
245
2.40
-0.26
β-agonist (puffs per day)
371
5.35
-1.65 *
241
5.78
-0.42
AM PEFR (L/min)
372
339.57
25.03 *
244
335.24
1.83
PM PEFR (L/min)
372
355.23
20.13 *
244
354.02
-0.49
Nocturnal Awakenings (#/week)
285
5.46
-2.03 *
195
5.57
-0.78
Both studies evaluated the effect of montelukast sodium on secondary outcomes, including asthma attack (utilization of health-care resources such as an unscheduled visit to a doctor's office, emergency room, or hospital; or treatment with oral, intravenous, or intramuscular corticosteroid), and use of oral corticosteroids for asthma rescue. In the Multinational study, significantly fewer patients (15.6% of patients) on montelukast sodium experienced asthma attacks compared with patients on placebo (27.3%, p < 0.001). In the US study, 7.8% of patients on montelukast sodium and 10.3% of patients on placebo experienced asthma attacks, but the difference between the two treatment groups was not significant (p = 0.334). In the Multinational study, significantly fewer patients (14.8% of patients) on montelukast sodium were prescribed oral corticosteroids for asthma rescue compared with patients on placebo (25.7%, p < 0.001). In the US study, 6.9% of patients on montelukast sodium and 9.9% of patients on placebo were prescribed oral corticosteroids for asthma rescue, but the difference between the two treatment groups was not significant (p = 0.196).
Onset of Action and Maintenance of Effects
In each placebo-controlled trial in adults, the treatment effect of montelukast sodium, measured by daily diary card parameters, including symptom scores, “as-needed” ß-agonist use, and PEFR measurements, was achieved after the first dose and was maintained throughout the dosing interval (24 hours). No significant change in treatment effect was observed during continuous once-daily evening administration in non-placebo-controlled extension trials for up to one year. Withdrawal of montelukast sodium in asthmatic patients after 12 weeks of continuous use did not cause rebound worsening of asthma.
Effects in Patients on Concomitant Inhaled Corticosteroids
Separate trials in adults evaluated the ability of montelukast sodium to add to the clinical effect of inhaled corticosteroids and to allow inhaled corticosteroid tapering when used concomitantly.
One randomized, placebo-controlled, parallel-group trial (n=226) enrolled adults with stable asthma with a mean FEV 1 of approximately 84% of predicted who were previously maintained on various inhaled corticosteroids (delivered by metered-dose aerosol or dry powder inhalers). The median age was 41.5 years (range 16 to 70); 52.2% were females and 47.8% were males.
The ethnic/racial distribution in this study was 92.0% Caucasian, 3.5% Black, 2.2% Hispanic, and 2.2% Asian. The types of inhaled corticosteroids and their mean baseline requirements included beclomethasone dipropionate (mean dose, 1203 mcg/day), triamcinolone acetonide (mean dose, 2004 mcg/day), flunisolide (mean dose, 1971 mcg/day), fluticasone propionate (mean dose, 1083 mcg/day), or budesonide (mean dose, 1192 mcg/day). Some of these inhaled corticosteroids were non-U.S.-approved formulations, and doses expressed may not be ex-actuator. The pre-study inhaled corticosteroid requirements were reduced by approximately 37% during a 5- to 7-week placebo run-in period designed to titrate patients toward their lowest effective inhaled corticosteroid dose. Treatment with montelukast sodium resulted in a further 47% reduction in mean inhaled corticosteroid dose compared with a mean reduction of 30% in the placebo group over the 12-week active treatment period (p ≤ 0.05). It is not known whether the results of this study can be generalized to patients with asthma who require higher doses of inhaled corticosteroids or systemic corticosteroids.
In another randomized, placebo-controlled, parallel-group trial (n=642) in a similar population of adult patients previously maintained, but not adequately controlled, on inhaled corticosteroids (beclomethasone 336 mcg/day), the addition of montelukast sodium to beclomethasone resulted in statistically significant improvements in FEV1 compared with those patients who were continued on beclomethasone alone or those patients who were withdrawn from beclomethasone and treated with montelukast sodium or placebo alone over the last 10 weeks of the 16-week, blinded treatment period. Patients who were randomized to treatment arms containing beclomethasone had statistically significantly better asthma control than those patients randomized to montelukast alone or placebo alone as indicated by FEV 1, daytime asthma symptoms, PEFR, nocturnal awakenings due to asthma, and “as-needed” ß-agonist requirements.
In adult patients with asthma with documented aspirin sensitivity, nearly all of whom were receiving concomitant inhaled and/or oral corticosteroids, a 4-week, randomized, parallel-group trial (n=80) demonstrated that montelukast sodium, compared with placebo, resulted in significant improvement in parameters of asthma control. The magnitude of effect of montelukast sodium in aspirin-sensitive patients was similar to the effect observed in the general population of asthma patients studied. The effect of montelukast sodium on the bronchoconstrictor response to aspirin or other non-steroidal anti-inflammatory drugs in aspirin-sensitive asthmatic patients has not been evaluated [see Warnings and Precautions (5.3)].
14.2 Exercise-Induced Bronchoconstriction (EIB)
Exercise-Induced Bronchoconstriction(Adults and Adolescents 15 years of age and older)
The efficacy of montelukast sodium, 10 mg, when given as a single dose 2 hours before exercise for the prevention of EIB was investigated in three (U.S. and Multinational), randomized, double-blind, placebo-controlled crossover studies that included a total of 160 adult and adolescent patients 15 years of age and older with EIB. Exercise challenge testing was conducted at 2 hours, 8.5 or 12 hours, and 24 hours following administration of a single dose of study drug (montelukast 10 mg or placebo). The primary endpoint was the mean maximum percent fall in FEV 1 following the 2 hours post-dose exercise challenge in all three studies (Study A, Study B, and Study C). In Study A, a single dose of montelukast sodium 10 mg demonstrated a statistically significant protective benefit against EIB when taken 2 hours prior to exercise. Some patients were protected from EIB at 8.5 and 24 hours after administration; however, some patients were not. The results for the mean maximum percent fall at each timepoint in Study A are shown in TABLE 3 and are representative of the results from the other two studies.
TABLE 3: Mean Maximum Percent Fall in FEV 1 Following Exercise Challenge in Study A (N=47) ANOVA Model
Time of exercise challenge following
medication administration
Mean Maximum percent fall in FEV 1*
Treatment difference % for
Montelukast sodium versus Placebo (95%CI) *
Montelukast
Placebo
2 hours
13
22
-9 (-12, -5)
8.5 hours
12
17
-5 (-9, -2)
24 hours
10
14
-4 (-7, -1)
*Least squares-mean
The efficacy of montelukast for prevention of EIB in patients below 6 years of age has not been established.
Daily administration of montelukast for the chronic treatment of asthma has not been established to prevent acute episodes of EIB.
In a 12-week, randomized, double-blind, parallel group study of 110 adult and adolescent asthmatics 15 years of age and older, with a mean baseline FEV 1 percent of predicted of 83% and with documented exercise-induced exacerbation of asthma, treatment with montelukast sodium, 10 mg, once daily in the evening, resulted in a statistically significant reduction in mean maximal percent fall in FEV 1 and mean time to recovery to within 5% of the pre-exercise FEV 1. Exercise challenge was conducted at the end of the dosing interval (i.e., 20 to 24 hours after the preceding dose). This effect was maintained throughout the 12-week treatment period indicating that tolerance did not occur. Montelukast sodium did not, however, prevent clinically significant deterioration in maximal percent fall in FEV 1 after exercise (i.e., ≥ 20% decrease from pre-exercise baseline) in 52% of patients studied. In a separate crossover study in adults, a similar effect was observed after two once-daily 10-mg doses of montelukast sodium.
14.3 Allergic Rhinitis (Seasonal and Perennial)
Seasonal Allergic Rhinitis
The efficacy of montelukast sodium tablets for the treatment of seasonal allergic rhinitis was investigated in 5 similarly designed, randomized, double-blind, parallel-group, placebo- and active-controlled (loratadine) trials conducted in North America. The 5 trials enrolled a total of 5029 patients, of whom 1799 were treated with montelukast sodium tablets. Patients were 15 to 82 years of age with a history of seasonal allergic rhinitis, a positive skin test to at least one relevant seasonal allergen, and active symptoms of seasonal allergic rhinitis at study entry.
The period of randomized treatment was 2 weeks in 4 trials and 4 weeks in one trial. The primary outcome variable was mean change from baseline in daytime nasal symptoms score (the average of individual scores of nasal congestion, rhinorrhea, nasal itching, sneezing) as assessed by patients on a 0-3 categorical scale.
Four of the five trials showed a significant reduction in daytime nasal symptoms scores with montelukast sodium 10-mg tablets compared with placebo. The results of one trial are shown below. The median age in this trial was 35.0 years (range 15 to 81); 65.4% were females and 34.6% were males. The ethnic/racial distribution in this study was 83.1% Caucasian, 6.4% other origins, 5.8% Black, and 4.8% Hispanic. The mean changes from baseline in daytime nasal symptoms score in the treatment groups that received montelukast tablets, loratadine, and placebo are shown in TABLE 5. The remaining three trials that demonstrated efficacy showed similar results.
TABLE 5: Effects of Montelukast on Daytime Nasal Symptoms Score* in a Placebo- and Active-controlled Trial in Patients with Seasonal Allergic Rhinitis (ANCOVA Model)
Treatment Group (N)
Baseline Mean Score
Mean Change
from Baseline
Difference Between Treatment and
Placebo (95% CI) Least-Squares Mean
Montelukast 10 mg (344)
2.09
-0.39
-0.13 †(-0.21, -0.06)
Placebo (351)
2.10
-0.26
N.A.
Active Control ‡(Loratadine 10 mg)(599)
2.06
-0.46
-0.24 † (-0.31, -0.17)
* Average of individual scores of nasal congestion, rhinorrhea, nasal itching, sneezing as assessed by patients on a 0-3 categorical scale.
† Statistically different from placebo (p≤0.001).
‡ The study was not designed for statistical comparison between montelukast sodium and the active control (loratadine).
Perennial Allergic Rhinitis
The efficacy of montelukast sodium tablets for the treatment of perennial allergic rhinitis was investigated in 2 randomized, double-blind, placebo-controlled studies conducted in North America and Europe. The two studies enrolled a total of 3357 patients, of whom 1632 received montelukast sodium 10-mg tablets. Patients 15 to 82 years of age with perennial allergic rhinitis as confirmed by history and a positive skin test to at least one relevant perennial allergen (dust mites, animal dander, and/or mold spores), who had active symptoms at the time of study entry, were enrolled.
In the study in which efficacy was demonstrated, the median age was 35 years (range 15 to 81); 64.1% were females and 35.9% were males. The ethnic/racial distribution in this study was 83.2% Caucasian, 8.1% Black, 5.4% Hispanic, 2.3% Asian, and 1.0% other origins. Montelukast sodium 10-mg tablets once daily was shown to significantly reduce symptoms of perennial allergic rhinitis over a 6-week treatment period (TABLE 6); in this study the primary outcome variable was mean change from baseline in daytime nasal symptoms score (the average of individual scores of nasal congestion, rhinorrhea, and sneezing).
TABLE 6: Effects of Montelukast Sodium on Daytime Nasal Symptoms Score* in a Placebo-controlled Trial in Patients with Perennial Allergic Rhinitis (ANCOVA Model)
Treatment Group (N)
Baseline Mean Score
Mean Change
from Baseline
Difference Between Treatment and
Placebo (95% CI) Least-Squares Mean
Montelukast 10 mg (1000)
2.09
-0.42
-0.08 † (-0.12, -0.04)
Placebo (980)
2.10
-0.35
N.A.
* Average of individual scores of nasal congestion, rhinorrhea, sneezing as assessed by patients on a 0-3 categorical scale.
†Statistically different from placebo (p ≤ 0.001).
The other 6-week study evaluated montelukast 10 mg (n=626), placebo (n=609), and an active-control (cetirizine 10 mg; n=120).
The primary analysis compared the mean change from baseline in daytime nasal symptoms score for montelukast vs. placebo over the first 4 weeks of treatment; the study was not designed for statistical comparison between montelukast sodium and the active-control. The primary outcome variable included nasal itching in addition to nasal congestion, rhinorrhea, and sneezing. The estimated difference between montelukast sodium and placebo was -0.04 with a 95% CI of (-0.09, 0.01). The estimated difference between the active-control and placebo was -0.10 with a 95% CI of (-0.19, -0.01).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Montelukast sodium 10-mg Film-Coated Tablets are beige, rounded square-shaped tablets, biconvex and debossed CL 26 on one side of the tablet and having plain surface on other side. They are supplied as follows:
NDC: 68071-4034-9 Bottles of 90
Storage
Store montelukast sodium 10-mg film-coated tablets at 20° to 25°C (68° to 77°F), excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from moisture and light. Store in original package. -
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
Information for Patients
- Patients should be advised to take montelukast daily as prescribed, even when they are asymptomatic, as well as during periods of worsening asthma, and to contact their physicians if their asthma is not well controlled.
- Patients should be advised that oral montelukast sodium is not for the treatment of acute asthma attacks. They should have appropriate short-acting inhaled ß-agonist medication available to treat asthma exacerbations. Patients who have exacerbations of asthma after exercise should be instructed to have available for rescue a short-acting inhaled ß-agonist. Daily administration of montelukast sodium for the chronic treatment of asthma has not been established to prevent acute episodes of EIB.
- Patients should be advised that, while using montelukast sodium, medical attention should be sought if short-acting inhaled bronchodilators are needed more often than usual, or if more than the maximum number of inhalations of short-acting bronchodilator treatment prescribed for a 24-hour period are needed.
- Patients receiving montelukast sodium should be instructed not to decrease the dose or stop taking any other anti-asthma medications unless instructed by a physician.
- Patients should be instructed to notify their physician if neuropsychiatric events occur while using montelukast sodium.
- Patients with known aspirin sensitivity should be advised to continue avoidance of aspirin or non-steroidal anti-inflammatory agents while taking montelukast.
Manufactured for:
Macleods Pharma USA, Inc.
Plainsboro, NJ 08536Manufactured by:
Macleods Pharmaceutical Ltd.
Baddi, Himachal Pradesh, INDIARevised : December 2016 #PM01747305
Patient Information
Montelukast sodium (MON-te-LOO-kast SOE-dee-um)Tablets
Read the Patient Information Leaflet that comes with montelukast sodium before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What are Montelukast sodium tablets?Montelukast sodium tablets are a prescription medicine that block substances in the body called leukotrienes. This may help to improve symptoms of asthma and allergic rhinitis. Montelukast sodium tablets do not contain a steroid.
Montelukast sodium tablets are used to:1.Prevent asthma attacks and for the long-term treatment of asthma in adults and adolescents ages 15 years and older.
Do not take montelukast sodium if you need relief right away for a sudden asthma attack. If you get an asthma attack, you should follow the instructions your healthcare provider gave you for treating asthma attacks.
2.Prevent exercise-induced asthma in people 15 years of age and older.
3.Help control the symptoms of allergic rhinitis (sneezing, stuffy nose, runny nose, itching of the nose). Montelukast sodium is used to treat:
outdoor allergies that happen part of the year (seasonal allergic rhinitis) in adults and adolescents ages 15 years and older, and
indoor allergies that happen all year (perennial allergic rhinitis) in adults and adolescents ages 15 years and older.
Who should not take Montelukast sodium tablets?
Do not take montelukast sodium tablets if you are allergic to any of its ingredients.
See the end of this leaflet for a complete list of the ingredients in montelukast sodium tablets.
What should I tell my healthcare provider before taking Montelukast sodium tablets?Before taking Montelukast sodium tablets, tell your healthcare provider if you:
are allergic to aspirin
have any other medical conditions
are pregnant or plan to become pregnant. Talk to your doctor if you are pregnant or plan to become pregnant, montelukast sodium may not be right for you.
are breast-feeding or plan to breast-feed. It is not known if montelukast sodium passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while taking montelukast sodium.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Some medicines may affect how montelukast sodium works, or montelukast sodium may affect how your other medicines work.
How should I take Montelukast sodium tablets?For anyone who takes montelukast tablets:
Take montelukast sodium tablets exactly as prescribed by your healthcare provider. Your healthcare provider will tell you how much montelukast tablets to take, and when to take it.
Do not stop taking montelukast sodium tablets or change when you take it without talking with your healthcare provider.
You can take montelukast sodium tablets with food or without food.
If you or your child misses a dose of montelukast sodium tablets, just take the next dose at your regular time. Do not take 2 doses at the same time.
If you take too much montelukast sodium, call your healthcare provider or a Poison Control Center right away
For adults and children 15 years of age and older with asthma:
Take montelukast sodium tablet 1 time each day, in the evening. Continue to take montelukast sodium tablet every day for as long as your healthcare provider prescribes it, even if you have no asthma symptoms.
Tell your healthcare provider right away if your asthma symptoms get worse, or if you need to use your rescue inhaler medicine more often for asthma attacks.
Do not take montelukast sodium tablet if you need relief right away from a sudden asthma attack. If you get an asthma attack, you should follow the instructions your healthcare provider gave you for treating asthma attacks.
Always have your rescue inhaler medicine with you for asthma attacks.
Do not stop taking or lower the dose of your other asthma medicines unless your healthcare provider tells you to.For patients 15 years of age and older for the prevention of exercise-induced asthma:
Take montelukast sodium tablet at least 2 hours before exercise.
Always have your rescue inhaler medicine with you for asthma attacks.
If you take montelukast sodium tablet every day for chronic asthma or allergic rhinitis, do not take another dose to prevent exercise-induced asthma. Talk to your healthcare provider about your treatment for exercise-induced asthma.
Do not take 2 doses of montelukast sodiun tablet within 24 hours (1 day).
For adults and children 15 years of age and older with seasonal allergic rhinitis, or for adults and children15 years of age and older with perennial allergic rhinitis:
Take montelukast sodium tablet 1 time each day, at about the same time each day.
What is the dose of montelukast sodium?
15 years and older: one 10-mg tablet.
What should I avoid while taking montelukast sodium?
If you have asthma and aspirin makes your asthma symptoms worse, continue to avoid taking aspirin or other medicines called nonsteroidal anti-inflammatory drugs (NSAIDs) while taking montelukast sodium.
What are the possible side effects of montelukast sodium?Montelukast sodium may cause serious side effects.
Behavior and mood-related changes. Tell your healthcare provider right away if you or your child have any of these symptoms while taking montelukast sodium:
agitation including aggressive behavior or hostility
irritability
attention problems
memory problems
bad or vivid dreams
restlessness
depression
sleep walking
disorientation (confusion)
suicidal thoughts and actions (including suicide)
feeling anxious
tremor
hallucinations (seeing or hearing things that are not really there)
trouble sleeping
uncontrolled muscle movements
Increase in certain white blood cells (eosinophils) and possible inflamed blood vessels throughout the body (systemic vasculitis). Rarely, this can happen in people with asthma who take montelukast sodium. This sometimes happens in people who also take a steroid medicine by mouth that is being stopped or the dose is being lowered.
Tell your healthcare provider right away if you get one or more of these symptoms:
a feeling of pins and needles or numbness of arms or legs
a flu-like illness
rash
severe inflammation (pain and swelling) of the sinuses (sinusitis)The most common side effects with montelukast sodium include:
upper respiratory infection
fever
headache
sore throat
cough
stomach pain
diarrhea
earache or ear infection
flu
runny nose
sinus infection
Other side effects with montelukast sodium include:
increased bleeding tendency, low blood platelet count
allergic reactions [including swelling of the face, lips, tongue, and/or throat (which may cause trouble breathing or swallowing), hives and itching]
dizziness, drowsiness, pins and needles/numbness, seizures (convulsions or fits)
palpitations
nose bleed, stuffy nose, swelling (inflammation) of the lungs.
heartburn, indigestion, inflammation of the pancreas, nausea, stomach or intestinal upset, vomiting
hepatitis
bruising, rash, severe skin reactions (erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis) that may occur without warning
joint pain, muscle aches and muscle crampsbedwetting in children
tiredness, swelling
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of montelukast tablets. For more information ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store montelukast sodium tablets?
Store montelukast sodium tablets at 68°-77°F (20°-25°C).
Keep montelukast sodium tablets in the container it comes in.
Keep montelukast sodium tablets in a dry place and away from light.
General Information about the safe and effective use of montelukast sodium tablets
Medicines are sometimes prescribed for purposes other than those mentioned in Patient Information Leaflets. Do not use montelukast sodium tablets for a condition for which it was not prescribed. Do not give montelukast sodium tablets to other people even if they have the same symptoms you have. It may harm them. Keep montelukast sodium tablets and all medicines out of the reach of children.
This leaflet summarizes information about montelukast sdium tablets. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about montelukast tablets that is written for health professionals. For more information, call Macleods Pharma USA, Inc., at 1-888-943-3210.
What are the ingredients in Montelukast Sodium tablets?
Active ingredient: montelukast sodium
Inactive ingredients: microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, hydroxypropyl cellulose, disodium edetate and magnesium stearate. The film coating contains: hydroxypropyl methylcellulose, hydroxypropyl cellulose, titanium dioxide, red ferric oxide and yellow ferric oxide.
Manufactured for:
Macleods Pharma USA, Inc.
Plainsboro, NJ 08536Manufactured by:
Macleods Pharmaceutical Ltd.
Baddi, Himachal Pradesh, INDIARevised:December,2016 PM01747305
- SPL PATIENT PACKAGE INSERT SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MONTELUKAST
montelukast tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68071-4034(NDC:33342-102) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MONTELUKAST SODIUM (UNII: U1O3J18SFL) (MONTELUKAST - UNII:MHM278SD3E) MONTELUKAST 10 mg Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) EDETATE DISODIUM (UNII: 7FLD91C86K) ISOPROPYL ALCOHOL (UNII: ND2M416302) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color orange (beige) Score no score Shape SQUARE (rounded sqaure) Size 8mm Flavor Imprint Code CL26 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68071-4034-9 90 in 1 BOTTLE; Type 0: Not a Combination Product 08/08/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203366 02/28/2012 Labeler - NuCare Pharmaceuticals,Inc. (010632300) Establishment Name Address ID/FEI Business Operations NuCare Pharmaceuticals,Inc. 010632300 relabel(68071-4034)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
