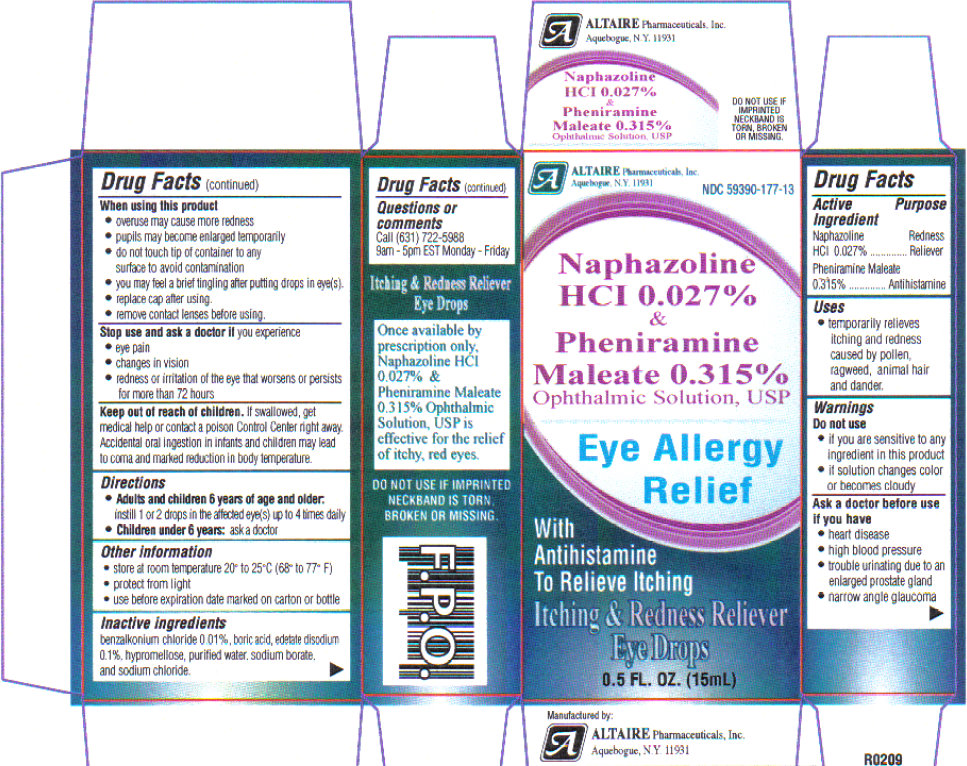
NAPHAZOLINE HCI AND PHENIRAMINE MALEATE- naphazoline hydrochloride , pheniramine maleate solution/ drops
Naphazoline HCI And Pheniramine Maleate by
Drug Labeling and Warnings
Naphazoline HCI And Pheniramine Maleate by is a Otc medication manufactured, distributed, or labeled by Altaire Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings: if you experience eye pain, changes in vision, continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours, discontinue use and consult a physician. Do not use in children under 6 years of age unless directed by a physician. If this solution changes color or becomes cloudy, do not use. Overuse of this product may produce increased redness of the eye.
If you are sensitive to any ingredient in this product, do not use. To avoid contamination, do not touch tip of container to any surface. Replace cap after using.
- DO NOT USE
- ASK DOCTOR
- WHEN USING
-
STOP USE
Stop use and ask a doctor if you experience: eye pain, changes in vision, redness or irritation of the eye that worsens or persists for more than 72 hours. Overuse of this product may produce increased redness of the eye. Pupils may become enlarged temporarily. You may experience a brief tingling sensation after putting drops in eyes.
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
SPL UNCLASSIFIED SECTION
Use before expiration date marked on the carton or bottle.
Available in 15mL NDC: 59390-177-13 and 30 mL NDC: 59390-177-18
- INACTIVE INGREDIENT
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NAPHAZOLINE HCI AND PHENIRAMINE MALEATE
naphazoline hydrochloride , pheniramine maleate solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59390-177 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPHAZOLINE HYDROCHLORIDE (UNII: MZ1131787D) (NAPHAZOLINE - UNII:H231GF11BV) NAPHAZOLINE HYDROCHLORIDE 0.27 mg in 1 mL PHENIRAMINE MALEATE (UNII: NYW905655B) (PHENIRAMINE - UNII:134FM9ZZ6M) PHENIRAMINE MALEATE 3.15 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BORIC ACID (UNII: R57ZHV85D4) EDETATE DISODIUM (UNII: 7FLD91C86K) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) WATER (UNII: 059QF0KO0R) SODIUM BORATE (UNII: 91MBZ8H3QO) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59390-177-13 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 10/06/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078208 10/06/2010 Labeler - Altaire Pharmaceuticals Inc. (786790378) Establishment Name Address ID/FEI Business Operations Altaire Pharmaceuticals Inc. 786790378 manufacture(59390-177)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.