AZESCHEW- ascorbic acid, cholecalciferol, thiamin, pyridoxine hydrochloride, folic acid, methylcobalamin, calcium citrate, ferrous fumerate, iodine bar, chewable
AzesChew by
Drug Labeling and Warnings
AzesChew by is a Other medication manufactured, distributed, or labeled by Basiem, LLC, Food Technology and Design,LLC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- HEALTH CLAIM:
-
DESCRIPTION:
AZESCHEW is an orally administered prescription vitamin formulation for the clinical dietary management of suboptimal nutritional status
in patients where advanced folate supplementation is required and nutritional supplementation in physiologically stressful conditions for maintenance of good health is needed.
AZESCHEW is a prescription dietary supplement for use throughout pregnancy, during the postnatal period for both lactating and non-lactating mothers, and throughout the childbearing years. AZESCHEW chews may be useful in improving the nutritional status of women prior to conception.
AZESCHEW should be administered under the supervision of a licensed medical practitioner. These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat,cure or prevent disease. AZESCHEW is manufactured in accordance with Current Good Manufacturing Practice for foods, using ingredients that have been approved by the U.S. Food and Drug Administration (FDA) as food additives or are “Generally Recognized as Safe” (GRAS) for their intended use. -
WARNING AND PRECAUTIONS
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately.
CONTRAINDICATIONS AZESCHEW chews are contraindicated in patients with a known hypersensitivity to any of the ingredients. Do not take this product if you are presently taking mineral oil, unless directed by a doctor.
PRECAUTION
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B is deficient. Folic acid in doses above 1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress. AZESCHEW chews should only be used under the direction and supervision of a licensed medical practitioner. - ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
-
HOW SUPPLIED HEALTH CLAIM:
AZESCHEW, Prenatal/Postnatal Support Dietary Supplement.
Chews are beige in color and individually wrapped in a strawberry colored foil. Each bottles contain 30 Chews 69597-353-30†
Dispensed by Prescription‡Manufactured in United States for:
Basiem, LLC
Madisonville, LA 70447Dispensed by Prescription†
MADE IN USA
Rev. 01/20†Solubiomix does not represent these product codes to be National Drug Codes (NDC). Product codes are formatted according to standard industry practice, to meet the formatting requirement by pedigree reporting and supply-chain control including pharmacies.
‡ This product is a prescription-folate with or without other dietary ingredients that – due to increased folate levels increased risk associated with masking of B12 deficiency (pernicious anemia) requires administration under the care of a licensed medical practitioner (61 FR 8760).1-3 The most appropriate way to ensure pedigree reporting consistent with these regulatory guidelines and safety monitoring is to dispense this product by prescription. This is not an Orange Book product. This product may be administered only under a physician’s supervision and all prescriptions using this product shall be pursuant to state statutes as applicable. The ingredients, indication or claims of this product are not to be construed to be drug claims.
1. Federal Register Notice of August 2, 1973 (38 FR 20750)
2. Federal Register Notice of October 17, 1980 (45 FR 69043, 69044)
3. Federal Register Notice of March 5, 1996 (61 FR 8760) - STORAGE AND HANDLING:
- PACKAGE LABEL:
-
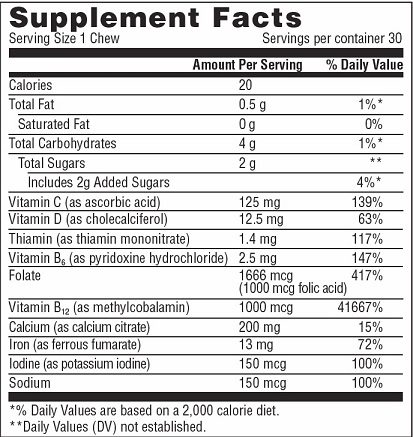
INGREDIENTS AND APPEARANCE
AZESCHEW
ascorbic acid, cholecalciferol, thiamin, pyridoxine hydrochloride, folic acid, methylcobalamin, calcium citrate, ferrous fumerate, iodine bar, chewableProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:69597-353 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 125 mg cholecalciferol (UNII: 1C6V77QF41) (cholecalciferol - UNII:1C6V77QF41) cholecalciferol 12.5 mg THIAMINE (UNII: X66NSO3N35) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 1.4 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 2.5 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg METHYLCOBALAMIN (UNII: BR1SN1JS2W) (METHYLCOBALAMIN - UNII:BR1SN1JS2W) METHYLCOBALAMIN 1 mg CALCIUM CITRATE ANHYDROUS (UNII: 86117BWO7P) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CITRATE ANHYDROUS 200 mg FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 13 mg POTASSIUM IODIDE (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) IODIDE ION .15 mg Inactive Ingredients Ingredient Name Strength SEA SALT (UNII: 87GE52P74G) STARCH, TAPIOCA (UNII: 24SC3U704I) RAW SUGAR (UNII: 8M707QY5GH) PALM OIL (UNII: 5QUO05548Z) NONI FRUIT JUICE (UNII: 6ULF85Z07J) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) CITRIC ACID ACETATE (UNII: DSO12WL7AU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:69597-353-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 01/07/2020 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 28 mm flavor scoring 1 Labeler - Basiem, LLC (079686680) Establishment Name Address ID/FEI Business Operations Food Technology and Design,LLC. 181426334 manufacture(69597-353)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.