PROMETHAZINE WITH CODEINE- promethazine hydrochloride and codeine phosphate solution
Promethazine with Codeine by
Drug Labeling and Warnings
Promethazine with Codeine by is a Prescription medication manufactured, distributed, or labeled by NuCare Pharmaceuticals,Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNING: RESPIRATORY DEPRESSION IN CHILDREN; DEATH RELATED TO ULTRA RAPID METABOLISM OF CODEINE TO MORPHINE and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Respiratory Depression in Children
The combination of promethazine hydrochloride and codeine phosphate is contraindicated in pediatric patients less than 6 years of age. Concomitant administration of promethazine products with other respiratory depressants has an association with respiratory depression, and sometimes death, in pediatric patients.Postmarketing cases of respiratory depression, including fatalities, have been reported with use of promethazine hydrochloride in pediatric patients less than 2 years of age. A wide range of weight-based doses of promethazine hydrochloride have resulted in respiratory depression in these patients.
Death Related to Ultra-Rapid Metabolism of Codeine to Morphine
Respiratory depression and death have occurred in children who received codeine following tonsillectomy and/or adenoidectomy and had evidence of being ultra-rapid metabolizers of codeine due to a CYP2D6 polymorphism.Risks from Concomitant Use with Benzodiazepines or Other CNS Depressants
Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death (see WARNINGS, PRECAUTIONS - Drug Interactions). Avoid use of opioid cough medications in patients taking benzodiazepines, other CNS depressants, or alcohol. -
DESCRIPTION
Each 5 mL (one teaspoonful), for oral administration contains: Promethazine hydrochloride 6.25 mg; codeine phosphate 10 mg in a flavored syrup base with a pH between 4.4 and 5.2. Alcohol 7%.
Inactive ingredients: Ascorbic acid, citric acid, FD&C blue #1, FD&C red #3, grape blend flavor, menthol, methylparaben, propylene glycol, propylparaben, purified water, saccharin sodium, sodium benzoate, sodium citrate and sucrose.
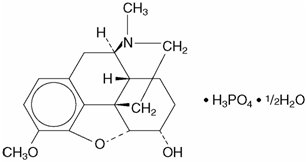
Codeine is one of the naturally occurring phenanthrene alkaloids of opium derived from the opium poppy, it is classified pharmacologically as a narcotic analgesic. Codeine phosphate may be chemically designated as 7,8-Didehydro-4, 5α-epoxy-3-methoxy-17-methylmorphinan-6α-ol phosphate (1:1) (salt) hemihydrate.
The phosphate salt of codeine occurs as white, needle-shaped crystals or white crystalline powder. Codeine phosphate is freely soluble in water and slightly soluble in alcohol. It has a molecular weight of 406.37, a molecular formula of C 18H 21NO 3H 3PO 4 ½ H 2O, and the following structural formula:
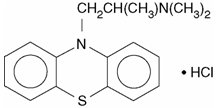
Promethazine hydrochloride, a phenothiazine derivative, is chemically designated as (±)-10-[2- (Dimethylamino)propyl] phenothiazine monohydrochloride.
Promethazine hydrochloride occurs as a white to faint yellow, practically odorless, crystalline powder which slowly oxidizes and turns blue on prolonged exposure to air. It is soluble in water and freely soluble in alcohol. It has a molecular weight of 320.88, a molecular formula of C 17H 20N 2SHCl, and the following structural formula:
-
CLINICAL PHARMACOLOGY
Codeine
Narcotic analgesics, including codeine, exert their primary effects on the central nervous system and gastrointestinal tract. The analgesic effects of codeine are due to its central action; however, the precise sites of action have not been determined, and the mechanisms involved appear to be quite complex. Codeine resembles morphine both structurally and pharmacologically, but its actions at the doses of codeine used therapeutically are milder, with less sedation, respiratory depression, and gastrointestinal, urinary, and pupillary effects. Codeine produces an increase in biliary tract pressure, but less than morphine or meperidine. Codeine is less constipating than morphine. Codeine has good antitussive activity, although less than that of morphine at equal doses. It is used in preference to morphine, because side effects are infrequent at the usual antitussive dose of codeine.
Codeine in oral therapeutic dosage does not usually exert major effects on the cardiovascular system.
Narcotic analgesics may cause nausea and vomiting by stimulating the chemoreceptor trigger zone (CTZ); however, they also depress the vomiting center, so that subsequent doses are unlikely to produce vomiting. Nausea is minimal after usual oral doses of codeine.
Narcotic analgesics cause histamine release, which appears to be responsible for wheals or urticaria sometimes seen at the site of injection on parenteral administration. Histamine release may also produce dilation of cutaneous blood vessels, with resultant flushing of the face and neck, pruritus, and sweating.
Codeine and its salts are well absorbed following both oral and parenteral administration. Codeine is about 2/3 as effective orally as parenterally. Codeine is metabolized primarily in the liver by enzymes of the endoplasmic reticulum, where it undergoes O-demethylation, N- demethylation, and partial conjugation with glucuronic acid. The drug is excreted primarily in the urine, largely as inactive metabolites and small amounts of free and conjugated morphine. Negligible amounts of codeine and its metabolites are found in the feces.
Following oral or subcutaneous administration of codeine, the onset of analgesia occurs within 15 to 30 minutes and lasts for four to six hours.
The cough-depressing action, in animal studies, was observed to occur 15 minutes after oral administration of codeine, peak action at 45 to 60 minutes after ingestion. The duration of action, which is dose-dependent, usually did not exceed 3 hours.
Promethazine
Promethazine is a phenothiazine derivative which differs structurally from the antipsychotic phenothiazines by the presence of a branched side chain and no ring substitution. It is thought that this configuration is responsible for its lack (1/10 that of chlorpromazine) of dopamine antagonist properties.
Promethazine is an H 1 receptor blocking agent. In addition to its antihistaminic action, it provides clinically useful sedative and antiemetic effects.
Promethazine is well absorbed from the gastrointestinal tract. Clinical effects are apparent within 20 minutes after oral administration and generally last four to six hours, although they may persist as long as 12 hours. Promethazine is metabolized by the liver to a variety of compounds; the sulfoxides of promethazine and N-demethylpromethazine are the predominant metabolites appearing in the urine.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
The combination of promethazine hydrochloride and codeine phosphate is contraindicated in pediatric patients less than 6 years of age, because the combination may cause fatal respiratory depression in this age population.
Codeine sulfate is contraindicated for post-operative pain management in children who have undergone tonsillectomy and/or adenoidectomy. (See WARNINGS - Death Related to Ultra- Rapid Metabolism of Codeine to Morphine).
Codeine is contraindicated in patients with a known hypersensitivity to the drug.
Promethazine hydrochloride is contraindicated in comatose states, and in individuals known to be hypersensitive or to have had an idiosyncratic reaction to promethazine or to other phenothiazines.
Antihistamines and codeine are both contraindicated for use in the treatment of lower respiratory tract symptoms, including asthma.
-
WARNINGS
(see Boxed Warnings)
Respiratory Depression in Children
The combination of promethazine hydrochloride and codeine phosphate is contraindicated in pediatric patients less than 6 years of age. Concomitant administration of promethazine products with other respiratory depressants has an association with respiratory depression, and sometimes death, in pediatric patients.
Postmarketing cases of respiratory depression, including fatalities, have been reported with use of promethazine hydrochloride in pediatric patients less than 2 years of age. A wide range of weight-based doses of promethazine hydrochloride have resulted in respiratory depression in these patients.
Respiratory depression leading to arrest, coma, and death has occurred with the use of codeine antitussives in young children, particularly in the under-one-year infants whose ability to deactivate the drug is not fully developed.
Codeine
-
Death Related to Ultra-Rapid Metabolism of Codeine to Morphine
Respiratory depression and death have occurred in children who received codeine in the post- operative period following tonsillectomy and/or adenoidectomy and had evidence of being ultra-rapid metabolizers of codeine (i.e., multiple copies of the gene for cytochrome P450 isoenzyme 2D6 [CYP2D6] or high morphine concentrations). Deaths have also occurred in nursing infants who were exposed to high levels of morphine in breast milk because their mothers were ultra-rapid metabolizers of codeine. (See PRECAUTIONS -Nursing Mothers).
Some individuals may be ultra-rapid metabolizers because of a specific CYP2D6 genotype (gene duplications denoted as *1/*1xN or *1/*2xN). The prevalence of this CYP2D6 phenotype varies widely and has been estimated at 0.5 to 1% in Chinese and Japanese, 0.5 to 1% in Hispanics, 1 to 10% in Caucasians, 3% in African Americans, and 16 to 28% in North Africans, Ethiopians, and Arabs. Data are not available for other ethnic groups. These individuals convert codeine into its active metabolite, morphine, more rapidly and completely than other people. This rapid conversion results in higher than expected serum morphine levels. Even at labeled dosage regimens, individuals who are ultra-rapid metabolizers may have life-threatening or fatal respiratory depression or experience signs of overdose (such as extreme sleepiness, confusion, or shallow breathing). (See OVERDOSAGE).
Children with obstructive sleep apnea who are treated with codeine for post-tonsillectomy and/or adenoidectomy pain may be particularly sensitive to the respiratory depressant effects of codeine that has been rapidly metabolized to morphine. Codeine is contraindicated for post-operative pain management in all pediatric patients undergoing tonsillectomy and/or adenoidectomy. (See CONTRAINDICATIONS).
When prescribing codeine-containing drugs, healthcare providers should choose the lowest effective dose for the shortest period of time and inform patients and caregivers about these risks and the signs of morphine overdose. (See OVERDOSAGE).
-
Risks from Concomitant Use with Benzodiazepines or Other CNS Depressants
Concomitant use of opioids, including Promethazine with Codeine Oral Solution, with benzodiazepines, or other CNS depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death . Because of these risks, avoid use of opioid cough medications in patients taking benzodiazepines, other CNS depressants, or alcohol. (See PRECAUTIONS - Drug Interactions).
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. Because of similar pharmacologic properties, it is reasonable to expect similar risk with concomitant use of opioid cough medications and benzodiazepines, other CNS depressants, or alcohol.
Advise both patients and caregivers about the risks of respiratory depression and sedation if Promethazine with Codeine Oral Solution is used with benzodiazepines, alcohol, or other CNS depressants. (See PRECAUTIONS – Information for Patients).
- Dosage of codeine SHOULD NOT BE INCREASED if cough fails to respond; an unresponsive cough should be reevaluated in 5 days or sooner for possible underlying pathology, such as foreign body or lower respiratory tract disease.
- Codeine may cause or aggravate constipation.
- Administration of codeine may be accomplished by histamine release and should be used with caution in atopic children.
-
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of narcotic analgesics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, intracranial lesions, or a preexisting increase in intracranial pressure. Narcotics may produce adverse reactions which may obscure the clinical course of patients with head injuries.
-
Asthma and Other Respiratory Conditions
Narcotic analgesics or cough suppressants, including codeine, should not be used in asthmatic patients (see CONTRAINDICATIONS). Nor should they be used in acute febrile illness associated with productive cough or in chronic respiratory disease where interference with ability to clear the tracheobronchial tree of secretions would have a deleterious effect on the patient’s respiratory function.
-
Hypotensive Effect
Codeine may produce orthostatic hypotension in ambulatory patients.
Promethazine
-
CNS Depression
Promethazine may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a vehicle or operating machinery. The impairment may be amplified by concomitant use of other central-nervous-system depressants such as alcohol, sedatives/hypnotics (including barbiturates), narcotics, narcotic analgesics, general anesthetics, tricyclic antidepressants, and tranquilizers; therefore avoid use of Promethazine with Codeine Oral Solution in patients on these medications. (See PRECAUTIONS – Information for Patients and Drug Interactions). -
Respiratory Depression
Promethazine may lead to potentially fatal respiratory depression.
Use of Promethazine in patients with compromised respiratory function (e.g. COPD, sleep apnea) should be avoided. -
Lower Seizure Threshold
Promethazine may lower seizure threshold. It should be used with caution in persons with seizure disorder or in persons who are using concomitant medications, such as narcotics or local anesthetics, which may also affect seizure threshold. -
Bone-Marrow Depression
Promethazine should be used with caution in patients with bone-marrow depression. Leukopenia and agranulocytosis have been reported, usually when promethazine hydrochloride has been used in association with other known marrow-toxic agents. -
Neuroleptic Malignant Syndrome
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with promethazine hydrochloride alone or in combination with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis and cardiac dysrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include 1) immediate discontinuation of promethazine hydrochloride, antipsychotic drugs, if any, and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
Since recurrences of NMS have been reported with phenothiazines, the reintroduction of promethazine hydrochloride should be carefully considered.
Use in Pediatric Patients
The combination of promethazine hydrochloride and codeine phosphate is contraindicated in pediatric patients less than 6 years of age. Concomitant administration of promethazine products with other respiratory depressants has an association with respiratory depression, and sometimes death, in pediatric patients. The association does not directly relate to individualized weight-based dosing, which might otherwise permit safe administration.
Respiratory depression and death have occurred in children with obstructive sleep apnea who received codeine in the post-operative period following tonsillectomy and/or adenoidectomy and had evidence of being ultra-rapid metabolizers of codeine (i.e., multiple copies of the gene for CYP2D6 or high morphine concentrations). These children may be particularly sensitive to the respiratory depressant effects of codeine that has been rapidly metabolized to morphine. Codeine is contraindicated for post-operative pain management in these patients. (See WARNINGS- Death Related to Ultra-Rapid Metabolism of Codeine to Morphine and CONTRAINDICATIONS).
Excessively large dosages of antihistamines, including promethazine hydrochloride, in pediatric patients may cause sudden death (see OVERDOSAGE). Hallucinations and convulsions have occurred with therapeutic doses and overdoses of promethazine hydrochloride in pediatric patients. In pediatric patients who are acutely ill associated with dehydration, there is an increased susceptibility to dystonias with the use of promethazine hydrochloride.
-
Death Related to Ultra-Rapid Metabolism of Codeine to Morphine
-
PRECAUTIONS
General
Narcotic analgesics, including codeine, should be administered with caution and the initial dose reduced in patients with acute abdominal conditions, convulsive disorders, significant hepatic or renal impairment, fever, hypothyroidism, Addison’s disease, ulcerative colitis, prostatic hypertrophy, in patients with recent gastrointestinal or urinary tract surgery, and in the very young or elderly or debilitated patients.
Drugs having anticholinergic properties should be used with caution in patients with narrow-angle glaucoma, prostatic hypertrophy, stenosing peptic ulcer, pyloroduodenal obstruction, and bladder-neck obstruction.
Promethazine should be used cautiously in persons with cardiovascular disease or with impairment of liver function.
Information for Patients
Patients should be advised to measure Promethazine with Codeine Oral Solution with an accurate measuring device. A household teaspoon is not an accurate measuring device and could lead to overdosage, especially when a half a teaspoon is measured. A pharmacist can recommend an appropriate measuring device and can provide instructions for measuring the correct dose.
Promethazine and codeine may cause marked drowsiness or may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a vehicle or operating machinery. Ambulatory patients should be told to avoid engaging in such activities until it is known that they do not become drowsy or dizzy from promethazine and codeine therapy. Pediatric patients should be supervised to avoid potential harm in bike riding or in other hazardous activities.
Inform patients and caregivers that potentially fatal additive effects may occur if Promethazine with Codeine Oral Solution is used with benzodiazepines or other CNS depressants, including alcohol. Because of this risk, patients should avoid concomitant use of Promethazine with Codeine Oral Solution with benzodiazepines or other CNS depressants, including alcohol. (See WARNINGS - Risks from Concomitant Use with Benzodiazepines or Other CNS Depressants) .
Patients should be advised to report any involuntary muscle movements.
Avoid prolonged exposure to the sun.
Codeine, like other narcotic analgesics, may produce orthostatic hypotension in some ambulatory patients. Patients should be cautioned accordingly.
Advise patients that some people have a genetic variation that results in codeine changing into morphine more rapidly and completely than other people. Most people are unaware of whether they are an ultra-rapid codeine metabolizer or not. These higher-than-normal levels of morphine in the blood may lead to life-threatening or fatal respiratory depression or signs of overdose such as extreme sleepiness, confusion, or shallow breathing. Children with this genetic variation who were prescribed codeine after tonsillectomy and/or adenoidectomy for obstructive sleep apnea may be at greatest risk based on reports of several deaths in this population due to respiratory depression. As a result, codeine is contraindicated in children who undergo tonsillectomy and/or adenoidectomy. Advise caregivers of children receiving codeine for other reasons to monitor for signs of respiratory depression. (See WARNINGS - Death Related to Ultra-Rapid Metabolism of Codeine to Morphine).
Nursing mothers taking codeine can also have higher morphine levels in their breast milk if they are ultra-rapid metabolizers. These higher levels of morphine in breast milk may lead to life-threatening or fatal side effects in nursing babies. Instruct nursing mothers to watch for signs of morphine toxicity in their infants including increased sleepiness (more than usual), difficulty breastfeeding, breathing difficulties, or limpness. Instruct nursing mothers to talk to the baby’s doctor immediately if they notice these signs and, if they can not reach the doctor right away, to take the baby to an emergency room or call 911 (or local emergency services).
Drug Interactions
Codeine
In patients receiving MAO inhibitors, an initial small test dose is advisable to allow observation of any excessive narcotic effects or MAOI interaction.The use of benzodiazepines, opioids, antihistamines, antipsychotics, anti-anxiety agents, or other CNS depressants (including alcohol) concomitantly with Promethazine Hydrochloride and Codeine Phosphate Oral Solution may cause an additive CNS depressant effect, profound sedation, respiratory depression, coma, and death and should be avoided. (See WARNINGS - Risks from Concomitant Use with Benzodiazepines or Other CNS Depressants) .
Promethazine
Epinephrine: Because of the potential for promethazine to reverse epinephrine’s vasopressor effect, epinephrine should NOT be used to treat hypotension associated with promethazine overdose.Anticholinergics: Concomitant use of other agents with anticholinergic properties should be undertaken with caution.
Monoamine Oxidase Inhibitors (MAOI): Drug interactions, including an increased incidence of extrapyramidal effects, have been reported when some MAOI and phenothiazines are used concomitantly.
Drug/Laboratory Test Interactions
Because narcotic analgesics may increase biliary tract pressure, with resultant increase in plasma amylase or lipase levels, determination of these enzyme levels may be unreliable for 24 hours after a narcotic analgesic has been given.
The following laboratory tests may be affected in patients who are receiving therapy with promethazine hydrochloride:
Pregnancy Tests: Diagnostic pregnancy tests based on immunological reactions between HCG and anti-HCG may result in false-negative or false-positive interpretations.
Glucose Tolerance Test: An increase in blood glucose has been reported in patients receiving promethazine.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to assess the carcinogenic potential of codeine or of promethazine, nor are there other animal or human data concerning carcinogenicity, mutagenicity, or impairment of fertility with these agents. Codeine has been reported to show no evidence of carcinogenicity or mutagenicity in a variety of test systems, including the micronucleus and sperm abnormality assays and the Salmonella assay.
Promethazine was nonmutagenic in the Salmonella test system of Ames.
Pregnancy
Teratogenic Effects
Pregnancy Category C:
Codeine: A study in rats and rabbits reported no teratogenic effect of codeine administered during the period of organogenesis in doses ranging from 5 to 120 mg/kg. In the rat, doses at the 120-mg/kg level, in the toxic range for the adult animal were associated with an increase in embryo resorption at the time of implantation. In another study a single 100-mg/kg dose of codeine administered to pregnant mice reportedly resulted in delayed ossification in the offspring.There are no studies in humans, and the significance of these findings to humans, if any, is not known.
Promethazine: Teratogenic effects have not been demonstrated in rat-feeding studies at doses of 6.25 and 12.5 mg/kg of promethazine hydrochloride. These doses are from approximately 2.1 to 4.2 times the maximum recommended total daily dose of promethazine for a 50-kg subject depending upon the indication for which the drug is prescribed. Daily doses of 25 mg/kg intraperitoneally have been found to produce fetal mortality in rats.
Specific studies to test the action of the drug on parturition, lactation, and development of the animal neonate were not done, but a general preliminary study in rats indicated no effect on these parameters. Although antihistamines have been found to produce fetal mortality in rodents, the pharmacological effects of histamine in the rodent do not parallel those in man. There are no adequate and well-controlled studies of promethazine in pregnant women.
Animal reproduction studies have not been conducted with the drug combination – promethazine and codeine. It is not known whether this drug combination can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Promethazine with Codeine Oral Solution should be given to a pregnant woman only if clearly needed.
Promethazine with Codeine Oral Solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
Dependence has been reported in newborns whose mothers took opiates regularly during pregnancy. Withdrawal signs include irritability, excessive crying, tremors, hyperreflexia, fever, vomiting, and diarrhea. Signs usually appear during the first few days of life.
Promethazine administered to a pregnant woman within two weeks of delivery may inhibit platelet aggregation in the newborn.
Labor and Delivery
Narcotic analgesics cross the placental barrier. The closer to the delivery and the larger the dose used, the greater the possibility of respiratory depression in the newborn. Narcotic analgesics should be avoided during labor if delivery of a premature infant is anticipated. If the mother has received narcotic analgesics during labor, newborn infants should be observed closely for signs of respiratory depression. Resuscitation may be required (see OVERDOSAGE). Limited data suggests that use of promethazine hydrochloride during labor and delivery does not have an appreciable effect on the duration of labor or delivery and does not increase the risk of need for intervention in the newborn.
The effect of promethazine and/or codeine on later growth and development of the newborn is unknown.
Nursing Mothers
Codeine is secreted into human milk. In women with normal codeine metabolism (normal CYP2D6 activity), the amount of codeine excreted into human milk is low and dose-dependent. Despite the common use of codeine products to manage postpartum pain, reports of adverse events in infants are rare. However, some women are ultra-rapid metabolizers of codeine. These women achieve higher-than-expected serum levels of codeine’s active metabolite, morphine, leading to higher-than-expected levels of morphine in breast milk and potentially dangerously high serum morphine levels in their breastfed infants. Therefore, maternal use of codeine can potentially lead to serious adverse reactions, including death, in nursing infants.
The risk of infant exposure to codeine and morphine through breast milk should be weighed against the benefits of breastfeeding for both the mother and baby. Caution should be exercised when codeine is administered to a nursing woman. If a codeine containing product is selected, the lowest dose should be prescribed for the shortest period of time to achieve the desired clinical effect. Mothers using codeine should be informed about when to seek immediate medical care and how to identify the signs and symptoms of neonatal toxicity, such as drowsiness or sedation, difficulty breastfeeding, breathing difficulties, and decreased tone, in their baby. Nursing mothers who are ultra-rapid metabolizers may also experience overdose symptoms such as extreme sleepiness, confusion or shallow breathing. Prescribers should closely monitor mother-infant pairs and notify treating pediatricians about the use of codeine during breastfeeding. (See WARNINGS - Death Related to Ultra-Rapid Metabolism of Codeine to Morphine.)
Pediatric Use
The combination of promethazine hydrochloride and codeine phosphate is contraindicated in pediatric patients less than 6 years of age, because the combination may cause fatal respiratory depression in this age population (see WARNINGS – Boxed Warning and Use in Pediatric Patients).
Respiratory depression and death have occurred in children with obstructive sleep apnea who received codeine in the post-operative period following tonsillectomy and/or adenoidectomy and had evidence of being ultra-rapid metabolizers of codeine (i.e., multiple copies of the gene for CYP2D6 or high morphine concentrations). These children may be particularly sensitive to the respiratory depressant effects of codeine that has been rapidly metabolized to morphine. Codeine is contraindicated for post-operative pain management in all pediatric patients undergoing tonsillectomy and/or adenoidectomy. (See WARNINGS - Death Related to Ultra-Rapid Metabolism of Codeine to Morphine and CONTRAINDICATIONS).
Geriatric Use
Clinical studies of Promethazine Hydrochloride and Codeine Phosphate Oral Solution did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
Sedating drugs may cause confusion and over-sedation in the elderly; elderly patients generally should be started on low doses of Promethazine with Codeine Oral Solution and observed closely.
-
ADVERSE REACTIONS
Codeine
Central Nervous System: CNS depression, particularly respiratory depression, and to a lesser extent circulatory depression; light-headedness, dizziness, sedation, euphoria, dysphoria, headache, transient hallucination, disorientation, visual disturbances, and convulsions.Cardiovascular: Tachycardia, bradycardia, palpitation, faintness, syncope, orthostatic hypotension (common to narcotic analgesics).
Gastrointestinal: Nausea, vomiting, constipation, and biliary tract spasm. Patients with chronic ulcerative colitis may experience increased colonic motility; in patients with acute ulcerative colitis, toxic dilation has been reported.
Genitourinary: Oliguria, urinary retention, antidiuretic effect has been reported (common to narcotic analgesics).
Allergic: Infrequent pruritus, giant urticaria, angioneurotic edema, and laryngeal edema.
Other: Flushing of the face, sweating and pruritus (due to opiate-induced histamine release); weakness.
Promethazine
Central Nervous System: Drowsiness is the most prominent CNS effect of this drug. Sedation, somnolence, blurred vision, dizziness, confusion, disorientation and extrapyramidal symptoms such as oculogyric crisis, torticollis, and tongue protrusion; lassitude, tinnitus, incoordination, fatigue, euphoria, nervousness, diplopia, insomnia, tremors, convulsive seizures, excitation, catatonic-like states, hysteria. Hallucinations have also been reported.Cardiovascular: Increased or decreased blood pressure, tachycardia, bradycardia, faintness.
Dermatologic: Dermatitis, photosensitivity, urticaria.
Hematologic: Leukopenia, thrombocytopenia, thrombocytopenic purpura, agranulocytosis.
Gastrointestinal: Dry mouth, nausea, vomiting, jaundice.
Respiratory: Asthma, nasal stuffiness, respiratory depression (potentially fatal) and apnea (potentially fatal) (see WARNINGS – Promethazine; Respiratory Depression).
Other: Angioneurotic edema. Neuroleptic malignant syndrome (potentially fatal) has also been reported (see WARNINGS – Promethazine; Neuroleptic Malignant Syndrome).
Paradoxical Reactions: Hyperexcitability and abnormal movements have been reported in patients following a single administration of promethazine hydrochloride. Consideration should be given to the discontinuation of promethazine hydrochloride and to the use of other drugs if these reactions occur. Respiratory depression, nightmares, delirium and agitated behavior have also been reported in some of these patients.
-
DRUG ABUSE AND DEPENDENCE
Abuse
Codeine is known to be subject to abuse; however, the abuse potential of oral codeine appears to be quite low. Even parenteral codeine does not appear to offer the psychic effects sought by addicts to the same degree as heroin or morphine. However, codeine must be administered only under close supervision to patients with a history of drug abuse or dependence.
-
OVERDOSAGE
Codeine
Serious overdose with codeine is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. The triad of coma, pinpoint pupils, and respiratory depression is strongly suggestive of opiate poisoning. In severe overdosage, particularly by the intravenous route, apnea, circulatory collapse, cardiac arrest, and death may occur. Promethazine is additive to the depressant effects of codeine.It is difficult to determine what constitutes a standard toxic or lethal dose. However, the lethal oral dose of codeine in an adult is reported to be in the range of 0.5 to 1 gram. Infants and children are believed to be relatively more sensitive to opiates on a body-weight basis. Elderly patients are also comparatively intolerant to opiates.
Promethazine
Signs and symptoms of overdosage with promethazine range from mild depression of the central nervous system and cardiovascular system to profound hypotension, respiratory depression, unconsciousness and sudden death. Other reported reactions include hyperreflexia, hypertonia, ataxia, athetosis and extensor-plantar reflexes (Babinski reflex).Stimulation may be evident, especially in children and geriatric patients. Convulsions may rarely occur. A paradoxical reaction has been reported in children receiving single doses of 75 mg to 125 mg orally, characterized by hyperexcitability and nightmares.
Atropine-like signs and symptoms – dry mouth, fixed dilated pupils, flushing, as well as gastrointestinal symptoms, may occur.
Treatment
Treatment of overdosage with promethazine and codeine is essentially symptomatic and supportive. Only in cases of extreme overdosage or individual sensitivity do vital signs including respiration, pulse, blood pressure, temperature, and EKG need to be monitored. Activated charcoal orally or by lavage may be given, or sodium or magnesium sulfate orally as a cathartic. Attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. The narcotic antagonist, naloxone hydrochloride, may be administered when significant respiratory depression occurs with promethazine and codeine; any depressant effects of promethazine are not reversed with naloxone. Diazepam may be used to control convulsions. Avoid analeptics, which may cause convulsions. Acidosis and electrolyte losses should be corrected. A rise in temperature or pulmonary complications may signal the need for institution of antibiotic therapy.Severe hypotension usually responds to the administration of norepinephrine or phenylephrine. EPINEPHRINE SHOULD NOT BE USED, since its use in a patient with partial adrenergic blockade may further lower the blood pressure.
Limited experience with dialysis indicates that it is not helpful.
-
HOW SUPPLIED
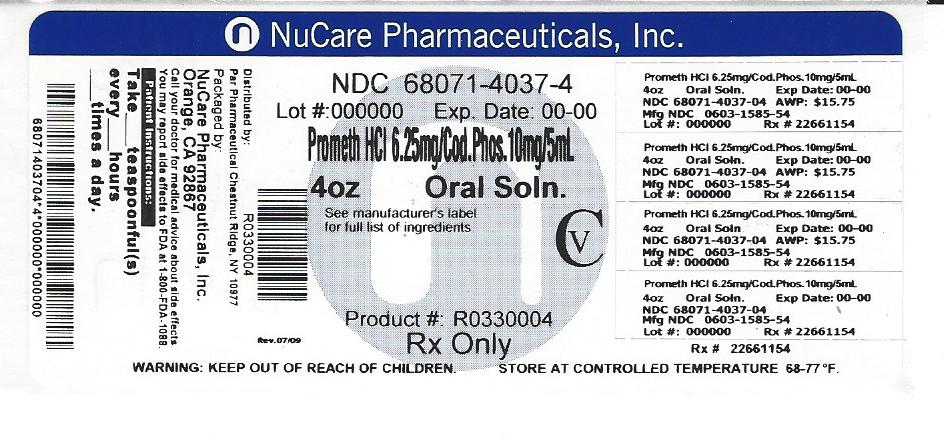
Promethazine with Codeine Oral Solution, a clear purple solution with odor of grape menthol, contains promethazine hydrochloride 6.25 mg/5 mL, codeine phosphate 10 mg/5 mL and alcohol 7 percent, and is available in 4 fluid ounce (120 mL) NDC: 68071-4037-4
-
DOSAGE AND ADMINISTRATION
It is important that Promethazine with Codeine Oral Solution is measured with an accurate measuring device (see PRECAUTIONS-Information for Patients). A household teaspoon is not an accurate measuring device and could lead to overdosage, especially when half a teaspoon is to be measured. It is strongly recommended that an accurate measuring device be used. A pharmacist can provide an appropriate device and can provide instructions for measuring the correct dose.
The combination of promethazine hydrochloride and codeine phosphate is contraindicated in pediatric patients less than 6 years of age, because the combination may cause fatal respiratory depression in this age population.
The average effective dose is given in the following table:
Adults (12 years of age and over) 5 mL (1 teaspoonful) every 4 to 6 hours, not to exceed 30.0 mL in 24 hours. Children 6 years to under 12 years 2.5 mL to 5 mL (½ to 1 teaspoonful) every 4 to 6 hours, not to exceed 30.0 mL in 24 hours.
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
MEDICATION GUIDE
Promethazine (proe meth’ a zeen) with Codeine (koe’ deen) Oral Solution C-V
(Promethazine Hydrochloride and Codeine Phosphate Oral Solution)What is the most important information I should know about Promethazine with Codeine Oral Solution?
- Taking Promethazine with Codeine Oral Solution with benzodiazepines, or other central nervous system depressants, including alcohol can cause severe drowsiness, breathing problems (respiratory depression), coma, and death.
- Promethazine with Codeine Oral Solution can cause you to be drowsy. Avoid driving a car or operating machinery during treatment with Promethazine with Codeine Oral Solution.
- Do not give Promethazine with Codeine Oral Solution to children who are under 6 years of age. It may harm them.
-
Do not give Promethazine with Codeine Oral Solution to a child to treat pain after tonsillectomy or adenoidectomy surgery.
- When you take Promethazine with Codeine Oral Solution, some of it changes into morphine in your body.
- In some children and adults this happens very quickly, and can cause you to stop breathing and cause death due to an overdose.
- Women who breastfeed should talk to their healthcare provider before taking Promethazine with Codeine Oral Solution.
- When you take Promethazine with Codeine Oral Solution, some of it changes into morphine in your body.
- In some women, this happens very quickly. Codeine and morphine pass into your breast milk. A large amount of morphine can cause your baby to die.
- Call your healthcare provider or get emergency medical help right away if anyone taking Promethazine with Codeine Oral Solution, or your breastfeeding baby has any of the symptoms below:
- increased sleepiness
- shallow breathing
- confusion
- limpness
- difficulty breathing
- your baby has difficulty breastfeeding
- Keep Promethazine with Codeine Oral Solution in a safe place away from children. Accidental use by a child is a medical emergency and can cause death. If a child accidentally takes Promethazine with Codeine Oral Solution, get emergency medical help right away.
- Promethazine with Codeine Oral Solution can cause serious side effects including death.
- Take Promethazine with Codeine Oral Solution exactly as prescribed by your healthcare provider. If you take the wrong dose of Promethazine with Codeine Oral Solution, you could overdose and die.
- It is especially important when you take Promethazine with Codeine Oral Solution that you know exactly what dose to take.
What is Promethazine with Codeine Oral Solution?
- Promethazine with Codeine Oral Solution is a prescription medicine used to temporarily treat cough and upper respiratory symptoms that you can have with allergies or a common cold. Promethazine with Codeine Oral Solution contains 2 medicines, promethazine and codeine. Promethazine is an antihistamine. Codeine is a narcotic cough suppressant.
- Promethazine with Codeine Oral Solution is a federal controlled substance (C-V) because it contains codeine that can be abused or lead to dependence. Keep Promethazine with Codeine Oral Solution in a safe place to prevent misuse and abuse. Selling or giving away Promethazine with Codeine Oral Solution may harm others, and is against the law. Tell your healthcare provider if you have ever abused or been dependent on alcohol, prescription medicines or street drugs.
- Promethazine with Codeine Oral Solution is not for children under 6 years of age.
Who should not take Promethazine with Codeine Oral Solution?
- Do not give Promethazine with Codeine Oral Solution to children who are under 6 years of age.
- Do not give Promethazine with Codeine Oral Solution to a child to treat pain after tonsillectomy or adenoidectomy surgery.
- Do not take Promethazine with Codeine Oral Solution if you are allergic to any of the ingredients in Promethazine with Codeine Oral Solution. See the end of this Medication Guide for a complete list of ingredients in Promethazine with Codeine Oral Solution.
- Do not take Promethazine with Codeine Oral Solution if you have asthma.
Before you take Promethazine with Codeine Oral Solution, tell your healthcare provider about all of your medical conditions, including if you:
- have a drug dependence
- drink alcohol
- have lung or breathing problems including chronic obstructive pulmonary disease (COPD), or sleep apnea
- have kidney or liver problems
- have thyroid problems including hypothyroidism
- have had a head injury
- have Addison’s disease
- have pain in your stomach-area (abdomen)
- have glaucoma (increased pressure in eyes)
- have a history of severe or persistent cough
- have diabetes
- have prostate problems
- have seizures
- have problems with your urinary tract (urethral stricture) or difficulty urinating
- have heart problems
- have blood disorders including low white blood cells
- have had surgery recently or plan to have surgery
- are pregnant or plan to become pregnant. It is not known if Promethazine with Codeine Oral Solution will harm your unborn baby. You and your healthcare provider should decide if you should take Promethazine with Codeine Oral Solution while you are pregnant.
- are breastfeeding or plan to breastfeed. Codeine Phosphate passes into your breast milk and may harm your baby. You and your healthcare provider should discuss whether you should take Promethazine with Codeine Oral Solution or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking Promethazine with Codeine Oral Solution with certain other medicines can cause side effects or affect how well Promethazine with Codeine Oral Solution or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider.
Especially tell your healthcare provider if you:
- take pain medicines such as narcotics
- take cold or allergy medicines that contain antihistamines or cough suppressants
- take medicines for mental illness (anti-psychotics, anti-anxiety)
- drink alcohol
- take medicines for depression, including monoamine oxidase inhibitors (MAOIs)
- take medicines for stomach or intestinal problems
Ask your healthcare provider if you are not sure if you take one of these medicines.
How should I take Promethazine with Codeine Oral Solution?
- Take Promethazine with Codeine Oral Solution exactly as your healthcare provider tells you to take it.
- Your healthcare provider will tell you how much Promethazine with Codeine Oral Solution to take and when to take it. Do not change your dose without talking to your healthcare provider.
- Ask your pharmacist to give you a measuring device to help you measure the correct amount of Promethazine with Codeine Oral Solution. Do not use a household teaspoon to measure your medicine. You may accidently take too much.
- Do not take more than 30 mL of Promethazine with Codeine Oral Solution in 24 hours.
- If you take too much Promethazine with Codeine Oral Solution, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking Promethazine with Codeine Oral Solution?
- Promethazine with Codeine Oral Solution can cause you to be drowsy. Avoid driving a car or operating machinery during treatment with Promethazine with Codeine Oral Solution. Also, watch your child closely when riding a bike.
- Avoid drinking alcohol during treatment with Promethazine with Codeine Oral Solution. Drinking alcohol can increase your chances of having serious side effects.
- Avoid spending a long time in sunlight.
What are the possible side effects of Promethazine with Codeine Oral Solution? Promethazine with Codeine Oral Solution may cause serious side effects, including:
- See “What is the most important information I should know about Promethazine with Codeine Oral Solution?”
- Breathing problems (respiratory depression) which can lead to death. Call your healthcare provider or get emergency treatment right away if you have excessive sleepiness, shallow or slow breathing, or confusion.
- Physical dependence or abuse. Take Promethazine with Codeine Oral Solution exactly as your healthcare provider tells you to take it. Stopping Promethazine with Codeine Oral Solution suddenly could cause withdrawal symptoms.
- Bowel problems including constipation or stomach pain.
- Increased intracranial pressure.
- Feeling dizzy, faint or light-headed, especially when you stand up (orthostatic hypotension). Light-headedness or fainting may happen when getting up too quickly from a sitting or lying position.
-
Neuroleptic malignant syndrome (NMS) which can lead to death. Tell you healthcare provider right away if you have any of the following symptoms of NMS:
- high fever
- confusion
- stiff muscles
- sweating
- changes in pulse, heart rate, and blood pressure
The most common side effects of Promethazine with Codeine Oral Solution include:
- sleepiness
- nausea and vomiting
- high blood pressure
- confusion
- difficulty urinating
- restlessness
- dryness of mouth, nose, throat
- trouble breathing
- fast heart beat
These are not all the possible side effects of Promethazine with Codeine Oral Solution.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Promethazine with Codeine Oral Solution?
- Store Promethazine with Codeine Oral Solution at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Promethazine with Codeine Oral Solution in a tightly closed, child-resistant container and out of the light.
- Safely throw away medicine that is out of date or no longer needed.
- Keep Promethazine with Codeine Oral Solution and all medicines out of the reach of children.
General information about the safe and effective use of Promethazine with Codeine Oral Solution.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Promethazine with Codeine Oral Solution for a condition for which it was not prescribed. Do not give Promethazine with Codeine Oral Solution to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Promethazine with Codeine Oral Solution that is written for health professionals.
What are the ingredients in Promethazine with Codeine Oral Solution?
Active ingredients: promethazine hydrochloride and codeine phosphate
Inactive ingredients: Ascorbic acid, citric acid, FD&C blue #1, FD&C red #3, grape blend flavor, menthol, methylparaben, propylene glycol, propylparaben, purified water, saccharin sodium, sodium benzoate, sodium citrate and sucrose.
Distributed by: Par Pharmaceutical, Chestnut Ridge, NY 10977
For more information, call 1-800-828-9393.This Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: 01/2017
R2
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PROMETHAZINE WITH CODEINE
promethazine hydrochloride and codeine phosphate solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68071-4037(NDC:0603-1585) Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROMETHAZINE HYDROCHLORIDE (UNII: R61ZEH7I1I) (PROMETHAZINE - UNII:FF28EJQ494) PROMETHAZINE HYDROCHLORIDE 6.25 mg in 5 mL CODEINE PHOSPHATE (UNII: GSL05Y1MN6) (CODEINE ANHYDROUS - UNII:UX6OWY2V7J) CODEINE PHOSPHATE 10 mg in 5 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) ASCORBIC ACID (UNII: PQ6CK8PD0R) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) MENTHOL (UNII: L7T10EIP3A) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) SUCROSE (UNII: C151H8M554) Product Characteristics Color Score Shape Size Flavor GRAPE (grape blend flavor) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68071-4037-4 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/09/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040650 01/31/2006 Labeler - NuCare Pharmaceuticals,Inc. (010632300) Establishment Name Address ID/FEI Business Operations NuCare Pharmaceuticals,Inc. 010632300 relabel(68071-4037)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.