SURPASS- diclofenac sodium cream
Surpass by
Drug Labeling and Warnings
Surpass by is a Animal medication manufactured, distributed, or labeled by Boehringer Ingelheim Animal Health USA Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Caution:
-
Description:
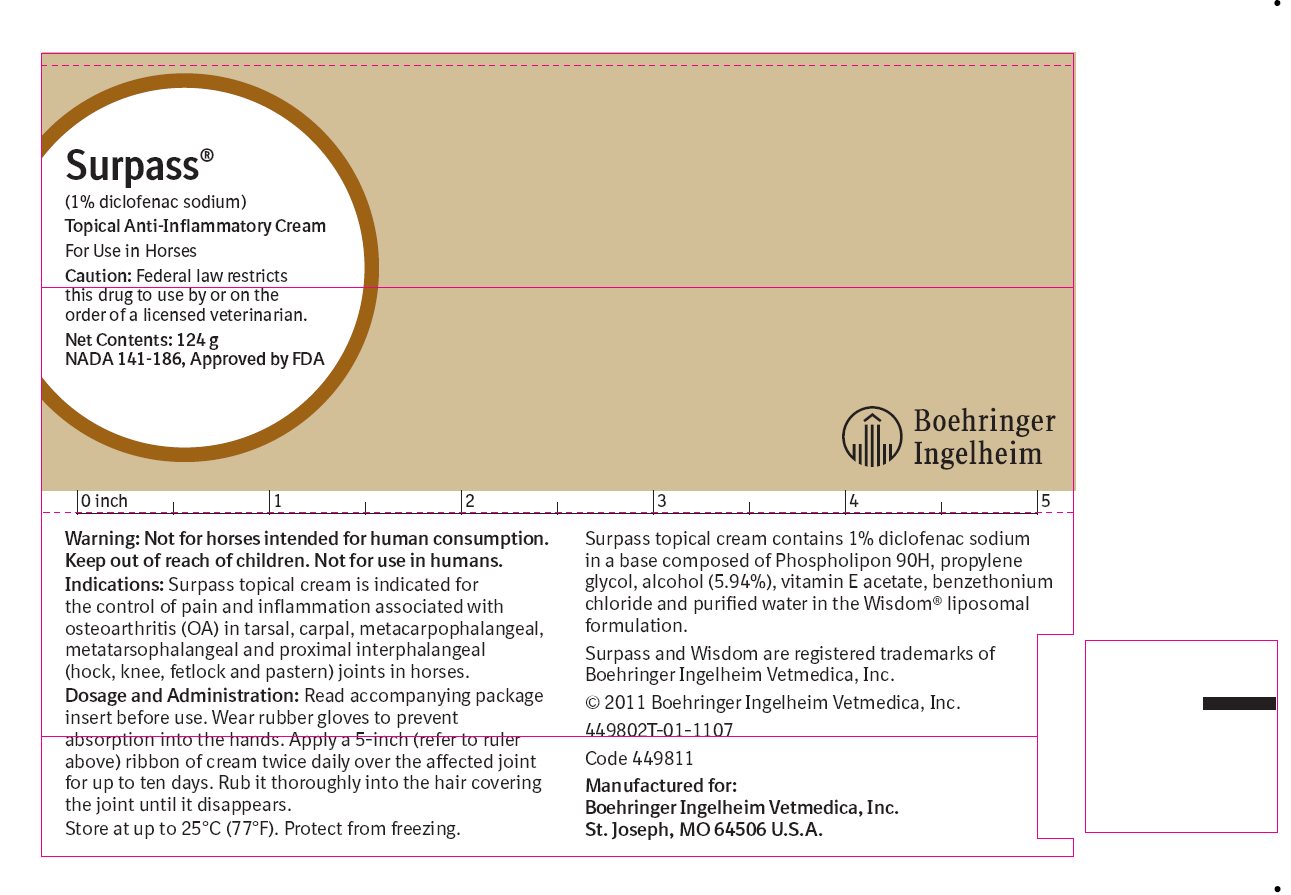
Surpass® topical cream contains 1% diclofenac sodium. Diclofenac is a non-steroidal anti-inflammatory drug (NSAID) of the phenylacetic acid class. The chemical name for diclofenac is sodium [o- (2,6-dichloroanilino)phenyl] acetate. The empirical formula is C14H10Cl2NNaO2 and the molecular weight is 318.13. Surpass topical cream contains 1% diclofenac sodium in a base composed of Phospholipon 90H, propylene glycol, alcohol (5.94%), vitamin E acetate, benzethonium chloride and purified water in the Wisdom® liposomal formulation.
- Indications:
-
Dosage and Administration:
Always provide the Client Information Sheet with the prescription.
Dosage: Apply a five-inch (5") ribbon of Surpass topical cream twice daily over the affected joint for up to ten days.
Administration: Wear rubber gloves to prevent absorption into the hands. Rub the cream thoroughly into the hair covering the joint until it disappears.
- Contraindications:
-
Warnings:
Not for horses intended for human consumption.
User Safety: Keep out of reach of children. Not for human use. Consult a physician in case of accidental ingestion by humans.
Wear gloves to prevent absorption into the hands. Direct contact with the skin should be avoided. If contact occurs, the skin should be washed immediately with soap and water.
Animal Safety: For topical use in horses only. Owners should be advised to observe for signs of potential drug toxicity (See Information for Owner or Person Treating Animal and Adverse Reactions).
-
Precautions:
Exceeding the recommended dosage or treating multiple joints may increase plasma concentrations of diclofenac (See Animal Safety). The systemic effects of excess diclofenac doses that exceed the recommended label amount and duration have not been evaluated.
Horses should undergo a thorough history and physical examination before initiation of NSAID therapy. Appropriate laboratory tests should be conducted to establish hematological and serum biochemical baseline data before and periodically during administration of any NSAID. Owners should be advised to observe for signs of potential drug toxicity (See Information for Owner or Person Treating Animal).
Treatment with Surpass cream should be terminated if signs such as inappetence, colic, fecal abnormalities, anemia or depression are observed.
As a class, NSAIDs may be associated with gastrointestinal and renal toxicity. When NSAIDs inhibit prostaglandins that cause inflammation, they may also inhibit prostaglandins that maintain normal homeostatic function. These anti-prostaglandin effects may result in clinically significant disease in patients with underlying or preexisting disease more often than in healthy patients. Patients at greatest risk for renal toxicity are those that are dehydrated, on concomitant diuretic therapy, or those with renal, cardiovascular and/or hepatic dysfunction.
Studies to determine the effect of Surpass topical cream when administered concomitantly with other drugs have not been conducted. Since many NSAIDs possess the potential to induce gastric ulceration, concomitant use of Surpass cream with any other anti-inflammatory drugs, such as other NSAIDs and corticosteroids, should be avoided. Drug compatibility should be monitored closely in patients receiving adjunctive therapy.
The safety of Surpass cream has not been investigated in breeding, pregnant or lactating horses, or in horses under one year of age.
-
Adverse Reactions:
During the field study, one diclofenac-treated horse developed colic on day four of the study and responded to symptomatic treatment. One placebo-treated horse exhibited mildly jaundiced mucous membranes on day five. Adverse reactions during the safety study included a gastric ulcer in one horse that received 5.6X the recommended dosage, diarrhea and uterine discharge in one horse that received 2.8X the recommended dosage, and weight loss in four of the six horses in the 5.6X dosage group.
To report suspected adverse reactions, to obtain a Material Safety Data Sheet or for technical assistance, call 1-866-638-2226.
-
Information for Owner or Person Treating Animal:
Owners should be advised of the potential for adverse reactions and be informed of the clinical signs associated with NSAID intolerance. Adverse reactions may include: weight loss, colic, diarrhea, or icterus. Serious adverse reactions associated with this drug class can occur without warning and, in rare situations, result in death. Owners should be advised to discontinue NSAID therapy and contact their veterinarian immediately if signs of intolerance are observed. The majority of patients with drug-related adverse reactions recover when the signs are recognized, drug administration is stopped, and veterinary care is initiated.
- Clinical Pharmacology:
-
Effectiveness:
In a controlled field study, 82 horses with osteoarthritis were treated with Surpass topical cream (42 horses) or placebo (40 horses). Lameness examinations were performed in horses with osteoarthritis associated with the tarsal, carpal, metacarpophalangeal, metatarsophalangeal and proximal interphalangeal joints. Investigators were masked to treatment. Investigators and owners were instructed to apply the test article over the affected joint twice daily (BID) for five days. Actual doses received by individual horses were calculated using tube weight measurements. The mean dose applied during the study was 73 mg per application. Average lameness scores showed statistically significant improvement following treatment with Surpass topical cream.
One diclofenac-treated horse developed colic and responded to symptomatic treatment on day four of the study. Day five bloodwork for the horse that colicked showed decreases in RBC, Hb and HCT, with an increase in PMNs, compared to pretreatment values. One placebo-treated horse exhibited mildly jaundiced mucous membranes on day five. No other adverse reactions were noted during the study.
-
Animal Safety:
A controlled safety study was conducted with Surpass topical cream. Four groups of six healthy adult horses received 0, 0.6, 1.7 or 2.8X the recommended daily dose for twenty-eight days. The daily dose was divided into two applications on day one of the study. For the remainder of the study, the entire daily dose was given at one time on 0, 1, 3 or 5 joints (tarsal, carpal, metacarpophalangeal, metatarsophalangeal, and proximal interphalangeal joints), depending on the dosage group. The control group of six horses was sham-dosed by rubbing the joints daily for twenty-eight days. An additional study group evaluated six horses that received 5.6X the recommended daily dose of Surpass topical cream distributed over five joints on a single day. This dose group was observed for fourteen days without additional treatment.
Clinical examinations, hematology, serum chemistry, synovial fluid analyses, gross necropsy and histopathology were performed. At necropsy, one horse in the 5.6X group had a glandular gastric ulcer. A horse in the 2.8X group had diarrhea and uterine discharge throughout the study. Four of the six horses in the 5.6X group lost weight during the study.
Dose-dependent increases in diclofenac plasma concentrations were detected in horses in the 1.7X and 2.8X treatment groups.
- Storage Information:
- How Supplied:
-
Client Information Sheet:
This summary contains important information about Surpass® topical cream. You should read this information before treating your horse with Surpass cream. This sheet of information is provided only as a summary and does not take the place of instructions from your veterinarian. Talk to your veterinarian if you have any questions about this information or to learn more about Surpass topical cream.
1. What is Surpass topical cream?
Surpass topical cream contains 1% diclofenac sodium. Diclofenac is a prescription non-narcotic, non-steroidal anti-inflammatory drug (NSAID) that controls pain. Diclofenac is used for the control of pain and inflammation associated with osteoarthritis (OA) in hock, knee, fetlock or pastern joints in horses.
2. What kind of results can I expect when my horse is being treated with Surpass cream?
Osteoarthritis is a painful condition caused by the progressive deterioration of the cartilage, accompanied by changes in the bone and soft tissues of the joint. This disease is characterized by pain and loss of function of the affected joint.
While Surpass topical cream is not a cure for osteoarthritis, it does control the pain and inflammation associated with OA and increases the horse’s mobility. The response to Surpass cream will vary from horse to horse. In most horses, maximum improvement is seen in less than one week.
3. Which horses should not receive treatment with Surpass topical cream?
Surpass cream is for topical use in horses only. Surpass cream should not be used in horses exhibiting allergic reactions to diclofenac. Surpass topical cream is not for use in horses intended for human consumption. The safety of Surpass cream has not been determined in horses less than one year of age, in horses used for breeding, pregnant mares, or mares nursing foals.
4. How do I apply Surpass topical cream to my horse?
Wear gloves to prevent absorption into the hands. Direct contact with the skin should be avoided. If contact occurs, the skin should be washed immediately with soap and water. Apply a five-inch (5") ribbon of Surpass cream twice daily over the affected joint for up to ten days. Rub it thoroughly into the hair covering the joint until it disappears.
5. What should I tell my veterinarian?
Tell your veterinarian if your horse has experienced allergic reactions to diclofenac or other medications. Tell your veterinarian if your horse is pregnant or nursing a foal, or if you intend to breed the horse. Tell your veterinarian if your horse has ever been diagnosed with an ulcer.
6. What possible side effects may occur in my horse’s therapy?
Horses should undergo a thorough history and physical examination by a veterinarian before the initiation of any Surpass therapy. Using more than the recommended amount of Surpass topical cream (for example, by treating multiple joints) has not been tested and is not recommended. Excessive doses to the skin have been shown to enter the bloodstream and this may increase the risk of side effects. Adverse reactions associated with NSAIDs may include: weight loss, colic, diarrhea, or yellowing of the gums, skin, or whites of the eyes (jaundice). Serious adverse reactions associated with this drug class can occur without warning and, in rare situations, result in death. Discontinue the use of Surpass cream and contact your veterinarian immediately if these signs are observed. The majority of patients with drug-related adverse reactions recover when the signs are recognized, drug administration is stopped, and veterinary care, if appropriate, is initiated.
7. What precautions should I take before administering Surpass topical cream?
Wear gloves to prevent absorption into the hands. Direct contact with the skin should be avoided. If contact occurs, the skin should be washed immediately with soap and water.
Surpass topical cream should only be applied to horses. Keep Surpass cream and all medications out of the reach of children. Surpass cream is not for human use. Contact a physician in case of accidental ingestion by people.
8. Can Surpass topical cream be given with other medications?
Surpass cream should not be given with any other anti-inflammatory drugs, such as other NSAIDs (for example, aspirin, phenylbutazone, flunixin) and corticosteroids (for example, cortisone, prednisone, dexamethasone, triamcinolone). Tell your veterinarian about all medicines that you are planning to administer in addition to Surpass cream. These should include other medications that you can obtain without a prescription.
9. How should I store Surpass topical cream?
Store at up to 25°C (77°F). Protect from freezing. Keep Surpass cream and all medications out of reach of children.
10. What else should I know about Surpass topical cream?
This document provides a summary of information about Surpass topical cream. If you have questions or concerns about Surpass cream or osteoarthritis pain, talk to your veterinarian.
As with all prescription medications, Surpass cream should only be administered to the patient for whom it was prescribed, and should only be used as prescribed.
To report a suspected adverse reaction, call 1-866-638-2226.
Surpass is a registered trademark of Boehringer Ingelheim Vetmedica, Inc.
Manufactured for:
Boehringer Ingelheim Vetmedica, Inc.
St. Joseph, MO 64506 U.S.A.
Manufactured under U.S. Patent Nos. 4,937,078 and 6,936,273.©2009 Boehringer Ingelheim Vetmedica, Inc. All Rights Reserved.
449804L-00-0901
- Principal Display Panel – Container Label, 124 g
- Principal Display Panel – Display Carton, 124 g
-
INGREDIENTS AND APPEARANCE
SURPASS
diclofenac sodium creamProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0010-4498 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DICLOFENAC SODIUM (UNII: QTG126297Q) (DICLOFENAC - UNII:144O8QL0L1) DICLOFENAC SODIUM 0.01 g in 1 g Inactive Ingredients Ingredient Name Strength LECITHIN, SOYBEAN (UNII: 1DI56QDM62) 0.10 g in 1 g .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) 0.01 g in 1 g PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 0.0495 g in 1 g ALCOHOL (UNII: 3K9958V90M) 0.0594 g in 1 g BENZETHONIUM CHLORIDE (UNII: PH41D05744) 0.0002 g in 1 g WATER (UNII: 059QF0KO0R) 0.77 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0010-4498-01 1 in 1 CARTON 1 124 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141186 05/20/2004 Labeler - Boehringer Ingelheim Vetmedica, Inc. (007134091)
Trademark Results [Surpass]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SURPASS 98244700 not registered Live/Pending |
Chongqing Sipasi E-Commerce Co., Ltd. 2023-10-28 |
 SURPASS 98042379 not registered Live/Pending |
Surpass Industry Co., Ltd. 2023-06-14 |
 SURPASS 97926504 not registered Live/Pending |
Shenzhen Muxing Zhichuang Technology Co., Ltd. 2023-05-08 |
 SURPASS 97712793 not registered Live/Pending |
CSL Behring L.L.C. 2022-12-12 |
 SURPASS 97637693 not registered Live/Pending |
Ray Beyond Behavioral Corp. 2022-10-18 |
 SURPASS 97637687 not registered Live/Pending |
Ray Beyond Behavioral Corp. 2022-10-18 |
 SURPASS 97637670 not registered Live/Pending |
Ray Beyond Behavioral Corp. 2022-10-18 |
 SURPASS 97637660 not registered Live/Pending |
Ray Beyond Behavioral Corp. 2022-10-18 |
 SURPASS 97529366 not registered Live/Pending |
BK Technologies, Inc. 2022-08-01 |
 SURPASS 97403839 not registered Live/Pending |
Standard Textile Co., Inc. 2022-05-10 |
 SURPASS 97079290 not registered Live/Pending |
WUXI YONGHONG TECHNOLOGY CO., LTD. 2021-10-18 |
 SURPASS 88699201 not registered Live/Pending |
USLBG 2019-11-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.