EULEXIN- flutamide capsule
Eulexin by
Drug Labeling and Warnings
Eulexin by is a Prescription medication manufactured, distributed, or labeled by Waylis Therapeutics LLC, Strides Pharma, Inc., Waylis Therapuetics LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNINGS
Hepatic Injury
There have been postmarketing reports of hospitalization and rarely death due to liver failure in patients taking Eulexin™. Evidence of hepatic injury included elevated serum transaminase levels, jaundice, hepatic encephalopathy and death related to acute hepatic failure. The hepatic injury was reversible after discontinuation of therapy in some patients. Approximately half of the reported cases occurred within the initial 3 months of treatment with Eulexin™.
Serum transaminase levels should be measured prior to starting treatment with Eulexin™. Eulexin™ is not recommended in patients whose ALT values exceed twice the upper limit of normal. Serum transaminase levels should then be measured monthly for the first 4 months of therapy, and periodically thereafter. Liver function tests also should be obtained at the first signs and symptoms suggestive of liver dysfunction, e.g., nausea, vomiting, abdominal pain, fatigue, anorexia, "flu-like" symptoms, hyperbilirubinuria, jaundice or right upper quadrant tenderness. If at any time, a patient has jaundice, or their ALT rises above 2 times the upper limit of normal, Eulexin™ should be immediately discontinued with close follow-up of liver function tests until resolution.
-
DESCRIPTION
Eulexin™ capsules contain flutamide, an acetanilid, nonsteroidal, orally active antiandrogen having the chemical name, ,,-trifluoro-2-methyl-4'-nitro-m-propionotoluidide.
Each capsule contains 125 mg flutamide. The compound is a buff to yellow powder with a molecular weight of 276.22 and the following structural formula:
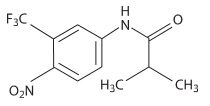
C11H11F3N2O3
In addition, each capsule contains the following inactive ingredients: corn starch, lactose monohydrate, magnesium stearate, povidone, and sodium lauryl sulfate. Gelatin capsule shells may contain gelatin, silicon dioxide, sodium lauryl sulfate, titanium dioxide, FDA/E172 Red Iron Oxide, FDA/E172 Yellow Iron Oxide, and black ink containing pharmaceutical glaze (modified) in SD-45, synthetic black iron oxide, N-butyl alcohol, SDA-3A alcohol, FD&C Blue No.2 Aluminum Lake, FD&C Red No.40 Aluminum Lake, FD&C Blue No.1 Aluminum Lake, and D&C Yellow No.10 Aluminum Lake.
-
CLINICAL PHARMACOLOGY
General
In animal studies, flutamide demonstrates potent antiandrogenic effects. It exerts its antiandrogenic action by inhibiting androgen uptake and/or by inhibiting nuclear binding of androgen in target tissues or both. Prostatic carcinoma is known to be androgen-sensitive and responds to treatment that counteracts the effect of androgen and/or removes the source of androgen, e.g., castration. Elevations of plasma testosterone and estradiol levels have been noted following flutamide administration.
Pharmacokinetics
Absorption
Analysis of plasma, urine, and feces following a single oral 200 mg dose of tritium-labeled Eulexin™ to human volunteers showed that the drug is rapidly and completely absorbed. Following a single 250 mg oral dose to normal adult volunteers, the biologically active alpha-hydroxylated metabolite reaches maximum plasma concentrations in about 2 hours, indicating that it is rapidly formed from flutamide. Food has no effect on the bioavailability of flutamide.
Distribution
In male rats administered an oral 5 mg/kg dose of 14C-flutamide neither flutamide nor any of its metabolites is preferentially accumulated in any tissue except the prostate. Total drug levels were highest 6 hours after drug administration in all tissues. Levels declined at roughly similar rates to low levels at 18 hours. The major metabolite was present at higher concentrations than Eulexin™ in all tissues studied. Following a single 250 mg oral dose to normal adult volunteers, low plasma concentrations of Eulexin™ were detected. The plasma half-life for the alpha-hydroxylated metabolite of Eulexin™ is approximately 6 hours. Eulexin™, in vivo, at steady-state plasma concentrations of 24 to 78 ng/mL, is 94% to 96% bound to plasma proteins. The active metabolite of Eulexin™, in vivo, at steady-state plasma concentrations of 1556 to 2284 ng/mL, is 92% to 94% bound to plasma proteins.
Metabolism
The composition of plasma radioactivity, following a single 200 mg oral dose of tritium-labeled Eulexin™ to normal adult volunteers, showed that Eulexin™ is rapidly and extensively metabolized, with Eulexin™ comprising only 2.5% of plasma radioactivity 1 hour after administration. At least six metabolites have been identified in plasma. The major plasma metabolite is a biologically active alpha-hydroxylated derivative which accounts for 23% of the plasma tritium 1 hour after drug administration. The major urinary metabolite is 2-amino-5nitro-4-(trifluoromethyl)phenol.
Excretion
Eulexin™ and its metabolites are excreted mainly in the urine with only 4.2% of a single dose excreted in the feces over 72 hours.
Plasma Pharmacokinetics of flutamide and Hydroxyflutamide in Geriatric Volunteers (mean ± SD) Single Dose flutamide Hydroxyflutamide Steady-State flutamide Hydroxyflutamide Cmax (ng/mL) 25.2 ± 34.2 894 ± 406 113 ± 213 1629 ± 586 Elimination half-life (hr) — 8.1 ± 1.3 7.8 9.6 ± 2.5 Tmax (hr) 1.9 ± 0.7 2.7 ± 1.0 1.3 ± 0.7 1.9 ± 0.6 Cmin (ng/mL) — — — 673 ± 316 Special Populations
Geriatric
Following multiple oral dosing of 250 mg t.i.d. in normal geriatric volunteers, Eulexin™ and its active metabolite approached steady-state plasma levels (based on pharmacokinetic simulations) after the fourth Eulexin™ dose. The half-life of the active metabolite in geriatric volunteers after a single Eulexin™ dose is about 8 hours and at steady-state in 9.6 hours.
Race
There are no known alterations in Eulexin™ absorption, distribution, metabolism, or excretion due to race.
Renal Impairment
Following a single 250 mg dose of Eulexin™ administered to subjects with chronic renal insufficiency, there appeared to be no correlation between creatinine clearance and either Cmax or AUC of Eulexin™. Renal impairment did not have an effect on the Cmax or AUC of the biologically active alpha-hydroxylated metabolite of Eulexin™. In subjects with creatinine clearance of < 29 mL/min, the half-life of the active metabolite was slightly prolonged. Eulexin™ and its active metabolite were not well dialyzed. Dose adjustment in patients with chronic renal insufficiency is not warranted.
Hepatic Impairment
No information on the pharmacokinetics of Eulexin™ in hepatic impairment is available (see BOXED WARNINGS, Hepatic Injury).
Drug-Drug Interactions
Interactions between Eulexin™ capsules and LHRH-agonists have not occurred. Increases in prothrombin time have been noted in patients receiving warfarin therapy (see PRECAUTIONS).
-
CLINICAL STUDIES
Eulexin™ has been demonstrated to interfere with testosterone at the cellular level. This can complement medical castration achieved with LHRH-agonists which suppresses testicular androgen production by inhibiting luteinizing hormone secretion.
The effects of combination therapy have been evaluated in two studies. One study evaluated the effects of Eulexin™ and an LHRH-agonist as neoadjuvant therapy to radiation in stage B2-C prostatic carcinoma and the other study evaluated Eulexin™ and an LHRH-agonist as the sole therapy in stage D2 prostatic carcinoma.
Stage B2-C Prostatic Carcinoma
The effects of hormonal treatment combined with radiation was studied in 466 patients (231 Eulexin™ capsules + goserelin acetate implant + radiation, 235 radiation alone) with bulky primary tumors confined to the prostate (stage B2) or extending beyond the capsule (stage C), with or without pelvic node involvement.
In this multicentered, controlled trial, administration of Eulexin™ capsules (250 mg t.i.d.) and goserelin acetate (3.6 mg depot) prior to and during radiation was associated with a significantly lower rate of local failure compared to radiation alone (16% vs. 33% at 4 years, P < 0.001). The combination therapy also resulted in a trend toward reduction in the incidence of distant metastases (27% vs. 36% at 4 years, P = 0.058). Median disease-free survival was significantly increased in patients who received complete hormonal therapy combined with radiation as compared to those patients who received radiation alone (4.4 vs 2.6 years, P < 0.001). Inclusion of normal PSA level as a criterion for disease-free survival also resulted in significantly increased median disease-free survival in patients receiving the combination therapy (2.7 vs. 1.5 years, P < 0.001).
Stage D2 Prostatic Carcinoma
To study the effects of combination therapy in metastatic disease, 617 patients (311 leuprolide + Eulexin™, 306 leuprolide + placebo) with previously untreated advanced prostatic carcinoma were enrolled in a large multicentered, controlled clinical trial.
Three and one-half years after the study was initiated, median survival had been reached. The median actuarial survival time was 34.9 months for patients treated with leuprolide and Eulexin™ versus 27.9 months for patients treated with leuprolide alone. This 7 month increment represents a 25% improvement in overall survival time with the Eulexin™ therapy. Analysis of progression-free survival showed a 2.6 month improvement in patients who received leuprolide plus Eulexin™, a 19% increment over leuprolide and placebo.
-
INDICATIONS AND USAGE
Eulexin™ capsules are indicated for use in combination with LHRH-agonists for the management of locally confined Stage B2-C and Stage D2 metastatic carcinoma of the prostate.
- CONTRAINDICATIONS
-
WARNINGS
Use in Women
Eulexin™ capsules are for use only in men. This product has no indication for women and should not be used in this population, particularly for nonserious or nonlife-threatening conditions.
Aniline toxicity
One metabolite of Eulexin™ is 4-nitro-3-fluoro-methylaniline. Several toxicities consistent with aniline exposure, including methemoglobinemia, hemolytic anemia and cholestatic jaundice have been observed in both animals and humans after Eulexin™ administration. In patients susceptible to aniline toxicity (e.g. persons with glucose-6-phosphate dehydrogenase deficiency, hemoglobin M disease and smokers), monitoring of methemoglobin levels should be considered.
-
PRECAUTIONS
General
In clinical trials, gynecomastia occurred in 9% of patients receiving Eulexin™ together with medical castration.
Information for Patients
Patients should be informed that Eulexin™ capsules and the drug used for medical castration should be administered concomitantly, and that they should not interrupt their dosing or stop taking these medications without consulting their physician.
Laboratory Tests
Regular assessment of serum Prostate Specific Antigen (PSA) may be helpful in monitoring the patient's response. If PSA levels rise significantly and consistently during Eulexin™ therapy the patient should be evaluated for clinical progression. For patients who have objective progression of disease together with an elevated PSA, a treatment period free of antiandrogen while continuing the LHRH analogue may be considered.
Drug Interactions
Increases in prothrombin time have been noted in patients receiving long-term warfarin therapy after Eulexin™ was initiated. Therefore close monitoring of prothrombin time is recommended and adjustment of the anticoagulant dose may be necessary when Eulexin™ capsules are administered concomitantly with warfarin.
Carcinogenesis and Mutagenesis and Impairment of Fertility
In a 1 year dietary study in male rats, interstitial cell adenomas of the testes were present in 49% to 75% of all treated rats (daily doses of 10, 30, and 50 mg/kg/ day were administered). These produced plasma Cmax values that are 1, 2, 3, and 4 fold respectively, those associated with therapeutic doses in humans. In male rats similarly dosed for 1 year, tumors were still present after 1 year of a drug-free period, but the incidences were 43% to 47%. In a 2 year carcinogenicity study in male rats, daily administration of Eulexin™ at these same doses produced testicular interstitial cell adenomas in 91% to 95% of all treated rats as opposed to 11% of untreated control rats. Mammary adenomas, adenocarcinomas, and fibroadenomas were increased in treated male rats at exposure levels that were 1 to 4 fold those observed during therapeutic dosing in humans. There are likewise reports of malignant breast neoplasms in men treated with Eulexin™ capsules (see ADVERSE REACTIONS section).
Eulexin™ did not demonstrate DNA modifying activity in the Ames Salmonella/ microsome Mutagenesis Assay. Dominant lethal tests in rats were negative. Reduced sperm counts were observed during a 6 week study of Eulexin™ mono-therapy in normal human volunteers.
Eulexin™ did not affect estrous cycles or interfere with the mating behavior of male and female rats when the drug was administered at 25 and 75 mg/kg/day prior to mating. Males treated with 150 mg/kg/day (30 times the minimum effective antiandrogenic dose) failed to mate; mating behavior returned to normal after dosing was stopped. Conception rates were decreased in all dosing groups. Suppression of spermatogenesis was observed in rats dosed for 52 weeks at approximately 3, 8, or 17 times the human dose and in dogs dosed for 78 weeks at 1.4, 2.3, and 3.7 times the human dose.
Animal Toxicology
Serious cardiac lesions were observed in 2/10 beagle dogs receiving 25 mg/kg/ day for 78 weeks and 3/16 receiving 40 mg/kg/day for 2 to 4 years. These lesions, indicative of chronic injury and repair processes, included chronic myxomatous degeneration, intra-atrial fibrosis, myocardial acidophilic degeneration, vasculitis and perivasculitis. The doses at which these lesions occurred were associated with 2-hydroxyflutamide levels that were 1 to 12 fold greater than those observed in humans at therapeutic levels.
Pregnancy
Pregnancy Category D
There was decreased 24 hour survival in the offspring of pregnant rats treated with Eulexin™ at doses of 30, 100 or 200 mg/kg/day (approximately 3, 9 and 19 times the human dose). A slight increase in minor variations in the development of the sternebrae and vertebrae was seen in fetuses of rats treated with two higher doses. Feminization of the male rats also occurred at the two higher dose levels. There was a decreased survival rate in the offspring of rabbits receiving the highest dose (15 mg/kg/day, equal to 1.4 times the human dose).
-
ADVERSE REACTIONS
Stage B2-C Prostatic Carcinoma
Treatment with Eulexin™ capsules and the goserelin acetate implant did not add substantially to the toxicity of radiation treatment alone. The following adverse experiences were reported during a multicenter clinical trial comparing Eulexin™ + goserelin acetate implant + radiation versus radiation alone. The most frequently reported (greater than 5%) adverse experiences are listed below:
Adverse Events During Acute Radiation Therapy (within first 90 days of radiation therapy) (n=231) Goserelin Acetate Implant + Eulexin™+ Radiation (n=235) Radiation Only % All % All Rectum/Large Bowel 80 76 Bladder 58 60 Skin 37 37 Adverse Events During Late Radiation Phase (after 90 days of radiation therapy) (n=231) Goserelin Acetate Implant + Eulexin™+ Radiation (n=235) Radiation Only % All % All Diarrhea 36 40 Cystitis 16 16 Rectal Bleeding 14 20 Proctitis 8 8 Hematuria 7 12 Additional adverse event data were collected for the combination therapy with radiation group over both the hormonal treatment and hormonal treatment plus radiation phases of the study. Adverse experiences occurring in more than 5% of patients in this group, over both parts of the study, were hot flashes (46%), diarrhea (40%), nausea (9%), and skin rash (8%).
Stage D2 Metastatic Carcinoma
The following adverse experiences were reported during a multicenter clinical trial comparing Eulexin™ + LHRH agonist versus placebo + LHRH agonist.
The most frequently reported (greater than 5%) adverse experiences during treatment with Eulexin™ capsules in combination with an LHRH agonist are listed in the table below. For comparison, adverse experiences seen with an LHRH agonist and placebo are also listed in the following table.
(n=294) Eulexin™+ LHRH agonist (n=28) Placebo + LHRH agonist % All % All Hot Flashes 61 57 Loss of Libido 36 31 Impotence 33 29 Diarrhea 12 4 Nausea/Vomiting 11 10 Gynecomastia 9 11 Other 7 9 Other GI 6 4 As shown in the table, for both treatment groups, the most frequently occurring adverse experiences (hot flashes, impotence, loss of libido) were those known to be associated with low serum androgen levels and known to occur with LHRH agonists alone.
The only notable difference was the higher incidence of diarrhea in the Eulexin™ + LHRH agonist group (12%), which was severe in 5% as opposed to the placebo + LHRH agonist (4%), which was severe in less than 1%.
In addition, the following adverse reactions were reported during treatment with Eulexin™ + LHRH agonist.
Cardiovascular System: hypertension in 1% of patients.
Central Nervous System: CNS (drowsiness/confusion/depression/anxiety/nervousness) reactions occurred in 1% of patients.
Gastrointestinal System: anorexia 4%, and other GI disorders occurred in 6% of patients.
Hematopoietic System: anemia occurred in 6%, leukopenia in 3%, and thrombocytopenia in 1% of patients.
Liver and Biliary System: hepatitis and jaundice in less than 1% of patients.
Skin: irritation at the injection site and rash occurred in 3% of patients.
Other: edema occurred in 4%, genitourinary and neuromuscular symptoms in 2%, and pulmonary symptoms in less than 1% of patients.
In addition, the following spontaneous adverse experiences have been reported during the marketing of Eulexin™: hemolytic anemia,macrocytic anemia,methemoglobinemia, sulfhemoglobinemia, photosensitivity reactions (including erythema, ulceration, bullous eruptions, and epidermal necrolysis) and urine discoloration. The urine was noted to change to an amber or yellow-green appearance which can be attributed to the Eulexin™ and/or its metabolites. Also reported were cholestatic jaundice, hepatic encephalopathy, and hepatic necrosis. The hepatic conditions were often reversible after discontinuing therapy; however, there have been reports of death following severe hepatic injury associated with use of Eulexin™.
Malignant breast neoplasms have occurred rarely in male patients being treated with Eulexin™ capsules.
-
OVERDOSAGE
In animal studies with Eulexin™ alone, signs of overdose included hypoactivity, piloerection, slow respiration, ataxia, and/or lacrimation, anorexia, tranquilization, emesis, and methemoglobinemia.
Clinical trials have been conducted with Eulexin™ in doses up to 1500 mg per day for periods up to 36 weeks with no serious adverse effects reported. Those adverse reactions reported included gynecomastia, breast tenderness, and some increases in SGOT. The single dose of Eulexin™ ordinarily associated with symptoms of overdose or considered to be life-threatening has not been established.
Eulexin™ is highly protein bound, and is not cleared by hemodialysis. As in the management of overdosage with any drug, it should be borne in mind that multiple agents may have been taken. If vomiting does not occur spontaneously, it should be induced if the patient is alert. General supportive care, including frequent monitoring of the vital signs and close observation of the patient, is indicated.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Eulexin™ capsules USP, 125 mg, are available as opaque, beige/beige capsules, imprinted "par/753" on the cap and body. They are available in bottle of 180 (NDC: 80725-600-18).
- SPL UNCLASSIFIED SECTION
-
INFORMATION FOR PATIENTS EULEXIN™ (FLUTAMIDE) CAPSULES USP RX ONLY
Important information for patients taking Eulexin™ capsules.
Read this information carefully each time your prescription is refilled because there may be new information available. This summary does not tell you everything you need to know about Eulexin™ therapy. Your doctor is the best source of information about your treatment. Ask your doctor about questions you have.
What is Eulexin™ therapy?
Eulexin™ capsules, in combination with other therapies, is a treatment option for men with some types of prostate cancer.
Prostate cancer results from the abnormal growth of prostate cells. Medical scientists do not know exactly what causes the abnormal cells, but age, environment, and genetics are important factors. Male hormones ("androgens") cause the cancer to grow. The cancer growth can be slowed down by blocking the effect of androgens.
The Eulexin™ product is used together with an injection called "LHRH agonist," as a combined treatment called "total androgen blockade." The goal of this treatment is to reduce androgen levels and to block the effect of androgen on the tumor. The LHRH agonist reduces androgen levels. Eulexin™ therapy blocks the effect of androgen on the tumor.
Who should not take the Eulexin™ product?
You should not take Eulexin™ capsules if you have liver problems or if you are allergic to it. Eulexin™ capsules are for use only in men; therefore women should not take Eulexin™ capsules.
Are there important risks I should know about Eulexin™ therapy?
Some men taking Eulexin™ had liver injury and needed to be hospitalized. In rare cases, men died because of liver failure while they were taking Eulexin™ capsules. In about half of these cases, the liver failure occurred in the first 3 months that they were taking Eulexin™ capsules.
Because the Eulexin™ product may cause liver failure, it is very important that you have all blood tests recommended by your doctor. These tests help identify whether you are having liver problems. A recommended schedule for these blood tests is:
- Before starting Eulexin™ treatment.
- Every month for the first 4 months of therapy.
- Periodically after the first 4 months.
In addition, you should call your doctor right away if you have any of the following signs or symptoms:
- Loss of appetite.
- Nausea and vomiting.
- Stomach or abdominal pain.
- Fatigue (feeling extremely tired).
- Flu-like symptoms (muscle aches, soreness).
- Brown urine.
- Jaundice (yellowing of the skin or whites of the eyes).
These may be signs of liver failure.
How should I take Eulexin™ capsules?
Take your Eulexin™ capsules as your doctor has prescribed. The usual dosing is 2 capsules every 8 hours.
Your doctor will determine whether Eulexin™ therapy is right for you based on many different factors. These include how large your tumor is, how far it has spread and your physical condition. In addition to Eulexin™ capsules, you may be getting other treatments, including regular injections of LHRH agonist or radiation therapy. Do not stop or interrupt any treatment without consulting your healthcare professional.
If you miss a dose of Eulexin™ capsules, simply continue therapy with your next scheduled dose. Do not try to make up for it by taking extra capsules.
Can I take other medicines?
If you are taking any other medicines, especially warfarin (a blood-thinning drug), tell your doctor before beginning Eulexin™ therapy.
What are the other possible side effects of taking Eulexin capsules?
In a medical study, when Eulexin™ capsules were taken together with an LHRH agonist, the most common side effects were hot flashes, loss of sex drive (libido) and impotence. In addition, some men had diarrhea, nausea or vomiting, and breast enlargement.
In another medical study, when the Eulexin™ product was taken together with goserelin acetate (an LHRH agonist) and radiation therapy, the side effects of Eulexin™ therapy were about the same as when radiation therapy was given alone. These included hot flashes, diarrhea, nausea and skin rash.
What can I do if I get diarrhea?
If you experience moderate diarrhea due to Eulexin™ capsules, the following advice may help:
- drink plenty of fluids
- reduce your intake of dairy products (for example, milk, cheese, yogurt).
- Increase your intake of whole grains, fruits and vegetables.
- Stop laxative use.
- Take nonprescription antidiarrheal medicines.
If your diarrhea continues or it becomes severe, contact your doctor right away.
Are there any other lab tests my doctor will be performing?
Your doctor may perform other regular tests (such as the PSA blood test) to ensure that your body is responding to treatment. Ask your doctor if you have any questions about how your Eulexin™ therapy is being monitored.
Please ask your doctor about any questions concerning prostate cancer or Eulexin™ therapy, or you can also ask for a more detailed leaflet that is written for healthcare professionals.
Manufactured for:
Waylis Therapeutics LLC
Wixom, MI 48393
844-200-7910Revised: 06/2021
- PRINCIPAL DISPLAY PANEL - 125 mg Capsule Bottle Label
-
INGREDIENTS AND APPEARANCE
EULEXIN
flutamide capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 80725-600 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUTAMIDE (UNII: 76W6J0943E) (FLUTAMIDE - UNII:76W6J0943E) FLUTAMIDE 125 mg Product Characteristics Color BROWN (Beige) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code 93;7120 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 80725-600-18 1 in 1 BOX 11/12/2021 1 180 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075298 11/12/2021 Labeler - Waylis Therapeutics LLC (117678921) Registrant - Strides Pharma, Inc. (118344504) Establishment Name Address ID/FEI Business Operations Waylis Therapuetics LLC 117678921 LABEL(80725-600)
Trademark Results [Eulexin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EULEXIN 90056265 not registered Live/Pending |
Woodward Pharma Services LLC 2020-07-16 |
 EULEXIN 73775571 1556519 Dead/Cancelled |
SCHERING CORPORATION 1989-01-19 |
 EULEXIN 73342623 1222342 Dead/Cancelled |
Schering Corporation 1981-12-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
